
The migration mechanisms of water and salt are critical for understanding the freeze-thaw (F-T) processes of saline soils.
This study conducted unidirectional open-system F-T tests on saturated clay specimens with varying chloride salt contents to explore the migration mechanisms of water and salt. Through Nuclear Magnetic Resonance (NMR) relaxation testing, the migration of water molecules was captured, providing valuable insights into the underlying mechanisms. The results show that the freezing process is highly dependent on the salt content. In specimens with low salt content, the driving force for water-salt migration is stronger. Because water and salt migration are driven by different mechanisms during F-T cycles, their migration occurs asynchronously.
Specifically, salt migration is influenced by both convection and diffusion, while water migration is primarily driven by convection.
This phenomenon is further corroborated by the redistribution patterns of water and salt after the F-T experiments. Additionally, it was found that frozen samples with varying salt contents but the same amount of thawed water exhibited similar water molecule mobility. Both matrix suction and water molecule mobility are dependent on thawed water content, and are not affected by salt content. Salt content has a significant impact on lowering the soil’s freezing point, which, in turn, influences matrix suction and water mobility.
Artificial ground freezing (AGF) technology is a construction technique that involves freezing soil by circulating refrigerants through pipes to prevent water penetration and increase soil strength. AGF is widely used in underground engineering and has strong potential for contaminant isolation. However, the application of AGF often leads to engineering issues such as frost damage. Additionally, soluble salts like NaCl, commonly found in coastal areas, lower the freezing point of soil.
Water and salt migration alter the soil’s pore structure and its compressibility, which is the primary cause of deformation after F-T cycles. During freezing, water migrates under osmotic pressure and the chemical energy of ice formation, while salt migrates under two mechanisms: either towards the freezing front or diffusing in the opposite direction due to concentration gradients.
Currently, there is limited in-depth research on the migration mechanisms of water and salt during the freezing and thawing of saline soils. This study continuously monitors samples during the F-T process to provide a deeper understanding of the migration mechanisms of water and salt in saline soils. A series of systematic F-T tests were performed using soils with different chloride salt contents. NMR (Nuclear Magnetic Resonance) relaxation testing was also conducted to characterize the mineral-water interactions and further explore the migration mechanisms of water and salt.
Sample Preparation:
The soil samples were collected from a coastal city in eastern China. Figure 1 shows the particle size distribution of the soil obtained using a laser particle analyzer (Microtrac S3500, USA).
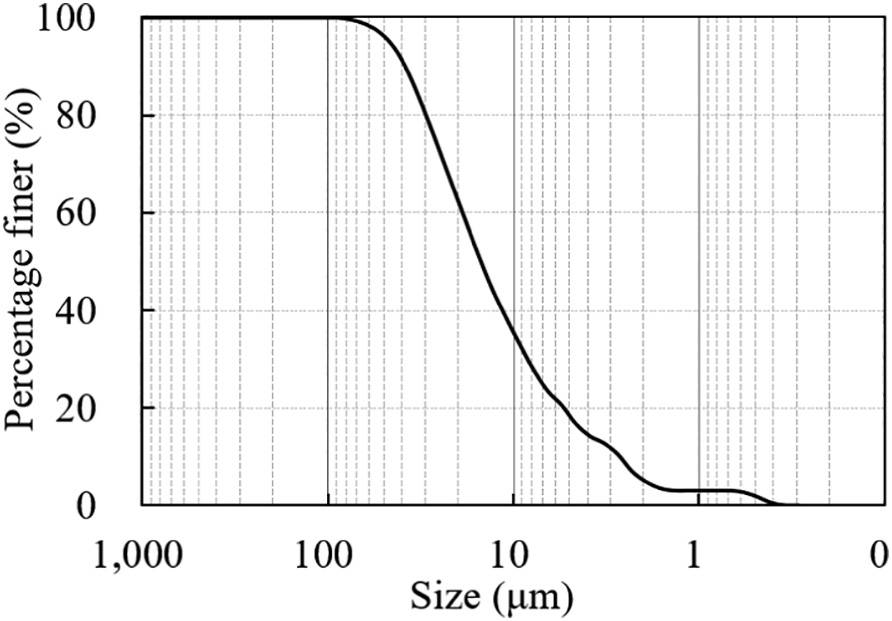
Figure 1: Particle Size Distribution of the Soil Samples
The soil was prepared according to the Chinese Soil Testing Method Standard (GB/T 50123-2019, 2019). The final sample dimensions were 20cm in diameter and 30cm in height (±0.3 cm) with a dry density of 1.65g/cm3. The samples were vacuum-saturated with NaCl solutions at concentrations of 0 mol/L, 0.64 mol/L, 1.32 mol/L, or 2.64 mol/L, corresponding to 0%, 1%, 2%, and 4% salt content. The sample preparation method and physical properties (such as density, water content, salt content, and saturation) for the NMR experiments were identical to the F-T tests, except the sample dimensions were 2.4cm in diameter and 5.5cm in height.
F-T Cycle Test Equipment:
Figure 2 shows the custom-designed F-T test apparatus. To simulate the freezing conditions in AGF engineering, the cold end was positioned at the bottom of the apparatus. Seven thermocouples and seven sensors were installed along the apparatus to monitor the temperature, thawed water content, volume conductivity, and matric suction during the F-T process.
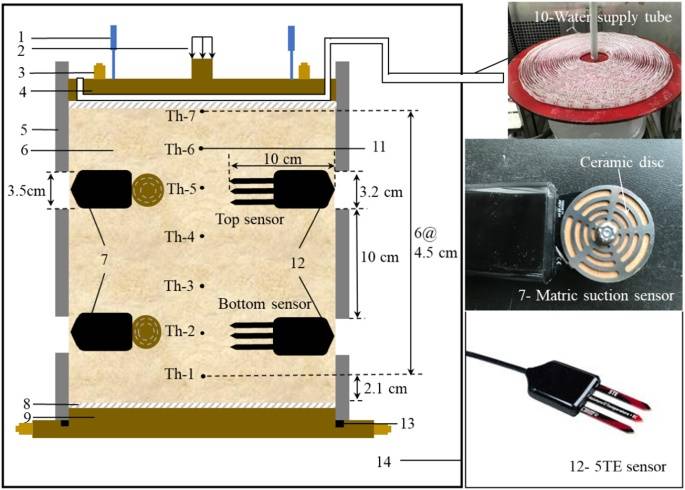
Figure 2: Diagram of the F-T Test Apparatus
Low-Field NMR Equipment:
The mobility of water molecules was monitored using a Nuclear Magnetic Resonance relaxation instrument produced by Niumag Analytical Instruments Co., Ltd., China, model: MesoMR23-060V-I.
Experimental Procedure:
1. After installing the sensors, the samples were pre-cooled in a 2°C environmental chamber for 12 hours to achieve hydraulic equilibrium between the ceramic disks and the surrounding soil.
2. The freezing process was then initiated, with the cold end set to -15°C and the warm end set to 2°C, while the environmental chamber maintained 2°C. The soil samples with different initial salt contents underwent freezing-thawing cycles.
3. The freezing process lasted for 60 hours to achieve thermal equilibrium, allowing the temperature at each monitoring point to stabilize.
4. Thawing was initiated by setting the environmental temperature to 25°C and disconnecting the cooling function. During the thawing process, pressure was applied to counteract friction between the sample and its container. During the F-T cycles, temperature, volume of thawed water, volume conductivity, and matric suction data were automatically recorded every 3 minutes.
5. After the F-T cycles, water and salt content were re-checked by drying and weighing samples taken from seven uniformly distributed points along the sample’s height.
NMR relaxation testing was performed to determine the mobility of water molecules during F-T, assisting in revealing the mechanisms of water and salt migration.
For the F-T experiments, the same soil samples were tested for NMR using the IR-CPMG sequence to measure longitudinal proton relaxation time (T1) and transverse proton relaxation time (T2) to produce 2D T1-T2 relaxation spectra, characterizing the interactions between protons. This method is an effective way to describe and evaluate pore water molecular mobility.
Heat Transfer:
The temperature variation at different locations within the samples is a crucial and direct indicator of the F-T process. Under the same temperature boundary conditions (cold end -15°C, warm end 2°C, with a temperature gradient of 0.57°C/cm along the sample height), the temperature patterns of samples with different salt contents were similar. For example, Figure 3 illustrates the temperature changes at different heights of a sample with 4% salt content during the F-T process. The three phases—pre-cooling, freezing, and thawing—are marked in the figure. Temperature stabilized by the 33rd hour and thawing began at the 72nd hour. Thawing occurred much faster than freezing due to heat transfer in all directions.
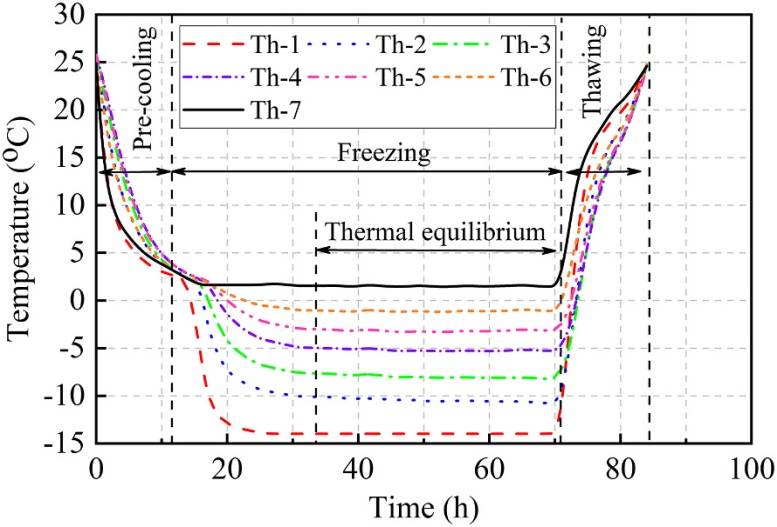
Figure 3: Temperature Variation at Different Heights of the Sample
The position of the freezing front is crucial as it separates the frozen and thawing zones. In thermal equilibrium, the freezing front is located through linear interpolation of the measured temperature distribution, according to the freezing point of the sample.
Previous studies have shown that during unidirectional freezing, the temperature distribution is linear near the cold end, but exhibits nonlinear behavior near the unsteady warm end. Therefore, the position of the unsteady freezing front is also determined through linear interpolation, as it is closer to the cold end during the early freezing stages. Figure 4 illustrates the evolution of the freezing front’s position, with its distance from the cold end changing over time. The freezing front moved quickly at the start of freezing and gradually reached thermal equilibrium by the end of the process. In the rapid-moving phase, the freezing front in samples with higher salt content moved slower than those with lower salt content due to the depression of the freezing point caused by the salt. In the stable phase, the final position of the freezing front depended on the salt content, with samples containing lower salt having the freezing front further from the cold end. Furthermore, salt migrated towards the cold end with the water, being excluded from the ice as the pore water froze. Therefore, salt concentration increased near the freezing front, further suppressing upward movement.
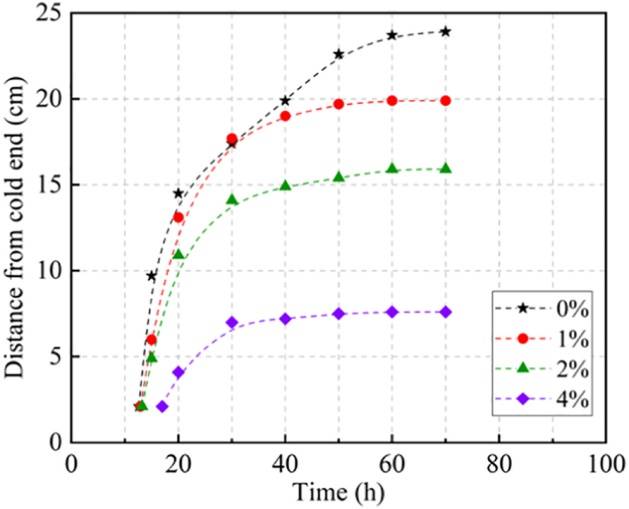
Figure 4: Evolution of the Freezing Front Position
Water Molecule Mobility:
As shown in Figure 5, low-field NMR T1-T2 test results were obtained for samples with two different salt contents at four temperatures to illustrate the representative stages of the T1-T2 spectra during F-T, as well as the influence of temperature and salt content. Specifically, four temperatures were chosen, as shown in Figure 5. For each sample, a temperature below the freezing point was selected (e.g., -3.5°C for 1% salt and -7.2°C for 2% salt) to analyze the effects of temperature and salt content on water molecule mobility. The T1/T2 ratio is a characteristic parameter indicating molecular mobility.
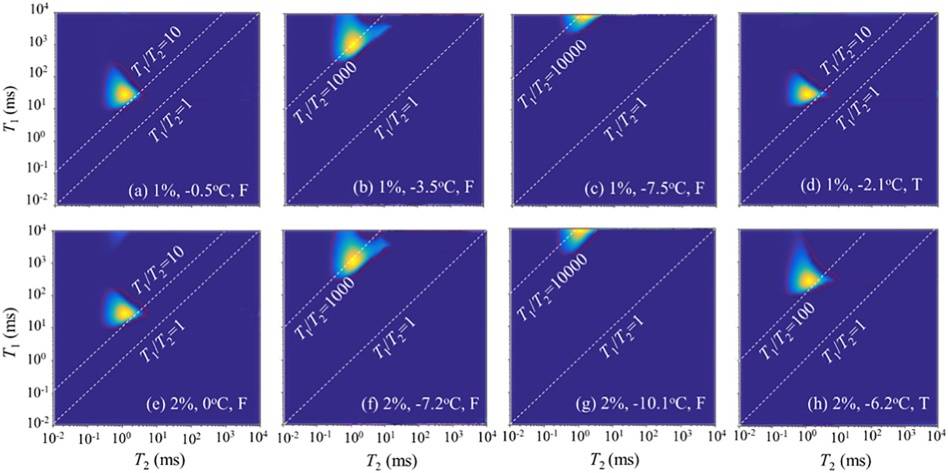
Figure 5: Low-field NMR T1-T2 2D NMR Spectra
This ratio can be used to explain the water-salt migration mechanism. During freezing, the T1/T2 ratio typically increases as temperature decreases and decreases as temperature increases, until full thawing occurs. From figures a and e, we can see that the T1/T2 peak ratio for soil samples with different salt contents is roughly the same, close to 10. This indicates that, in the thawed state, the T1/T2 ratio is independent of salt content. Similarly, during freezing, by comparing figures b and f, the T1/T2 peak ratio for both samples is around 1000. This further suggests that the solubility of salts does not directly affect the mobility of water molecules. Additionally, higher salt content lowers the freezing point, thus affecting the water content post-thaw and subsequently influencing water molecule mobility during the F-T process.
Systematic F-T tests were performed on clay samples saturated with different concentrations of sodium chloride solution to study the migration mechanisms of water and salt during the freeze-thaw cycles. The main conclusions are as follows:
[1] Liu J, Yang P, Yang Z J. Water and salt migration mechanisms of saturated chloride clay during freeze-thaw in an open system. Cold Regions Science and Technology, 2021, 186(8):103277.

Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top