Abstract:
This study developed a 3D printed food emulsion stabilized by three emulsifiers: Whey Protein Isolate (WPI), Hydroxypropyl Starch (HS), and Carrageenan. Low-field nuclear magnetic resonance (LF-NMR) technology was used to investigate the proton migration of hydrogen nuclei in the emulsion stabilized by WPI, HS, and Carrageenan. First, the T2 spectra of individual WPI, HS, and Carrageenan suspensions were analyzed, revealing that, in addition to the hydrogen protons of bound water, immobile water, and free water, the T2 distribution in WPI and HS suspensions also showed hydrogen protons associated with monolayer water. At the same time, an increase in the concentrations of WPI, HS, and Carrageenan reduced the water mobility. Furthermore, analysis of the T2 spectra of the stable emulsions mixed with WPI, HS, and Carrageenan showed that the free water proton signal was closely related to the concentration of these emulsifiers. Increasing the WPI concentration from 2% to 6%, or adding relatively low concentrations of HS and Carrageenan to WPI-stabilized emulsions, enhanced the stability of the WPI-stabilized emulsion system by restricting the movement of water molecules.
Research Background:
Three-dimensional (3D) printed custom foods are an emerging technology that enables personalized food products tailored to individual tastes, colors, and textures. A variety of 3D food printing materials have been developed, including fruit and vegetable-based materials (such as strawberry, mashed potatoes), protein-based materials (such as whey protein isolate, surimi), and starch-based materials (such as cookie dough). Most 3D food printing is based on extrusion printing, and studies have explored the printability of food materials in terms of rheological properties. However, little research has been conducted on how the interactions between different types of materials (e.g., water, lipids) affect the liquid migration rate in the resulting formulations, and how these interactions reveal the stability mechanisms of these formulations.
Emulsions, as immiscible stable mixtures of water, lipids, and emulsifiers, are often challenging to mix into a stable homogeneous state with the desired rheological properties. Research on developing emulsion-based 3D printing food formulations is scarce. Furthermore, studies on how food colloid interactions influence water and fat migration in emulsion-based 3D printing formulations are relatively limited. Low-field nuclear magnetic resonance (LF-NMR), a fast and non-destructive method, reveals the state and movement of water molecules in food by determining hydrogen proton relaxation times. It provides insights into water distribution, water-oil content, and the interactions between oil and hydrophilic colloid molecules in emulsions. This paper uses LF-NMR to assess the complex 3D printed food emulsion formulations.
Sample Preparation:
Whey protein isolate (WPI), hydroxypropyl starch (HS), and carrageenan were purchased from respective suppliers. Soybean oil was purchased from a local supermarket.
Concentrations of 2.0%, 4.0%, and 6.0% (w/v) WPI and HS, and 0.5%, 1.0%, and 2.0% (w/v) carrageenan were added to distilled water, respectively. After continuous heating and stirring, the resulting sample names were WPI2%, WPI4%, WPI6%, HS2%, HS4%, HS6%, Ca0.5%, Ca1%, and Ca2%, representing suspensions of WPI (2%, 4%, 6%), HS (2%, 4%, 6%), and carrageenan (0.5%, 1%, 2%).
WPI, HS, and carrageenan concentrations ranging from 0.5-6.0% (w/v) were added to distilled water. The mixture was stirred and heated, then combined with preheated soybean oil and the water suspension, followed by high-speed homogenization and continuous heating and stirring. This resulted in emulsions containing 20% oil at varying concentrations of WPI, HS, and carrageenan.
The corresponding emulsion sample names are listed in Table 1.
Low-Field NMR Testing Equipment:
The migration characteristics of hydrogen protons were monitored by an NMR relaxation measurement instrument, manufactured by Suzhou Niumag Analytical Instruments Co., Ltd., model: PQ001.
Experimental Procedure:
1. Approximately 5 g of the sample was placed into a 25 mm NMR sample tube covered with a Teflon film and inserted into the instrument.
2. T2 measurements were conducted at 25°C using the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence, with a wait time of 2000 ms, echo time of 0.200 ms, a total of 8000 echoes, and 4 repeat scans. The CPMG decay curve was fitted using the instrument’s inversion software.
3. Based on the above analysis, T2 peak positions, peak areas (A2), and relative percentages for each peak were calculated. Additionally, the time point of maximum curvature (Tcur) was defined as the time required for the decay curve to reach maximum curvature, reflecting the sample’s decay rate.
4. The single-component relaxation time (T2W) was recorded, which is approximately the time required for the signal decay to reach 40%.
5. Measurements were repeated three times.
Results and Analysis:
Single-component Emulsifier Suspensions:
Based on the T2 decay curves of 2%, 4%, and 6% single-component emulsifier suspensions, T2W and Tcur were obtained. As the concentration increased from 2% to 6%, both T2W and Tcur decreased, indicating faster decay processes and stronger binding of hydrogen protons. This shows that by increasing the emulsifier concentration, the molecular interactions within or between the emulsifier molecules are affected, thus increasing their binding capacity with hydrogen protons.
In the 2% WPI and HS suspensions, three relaxation signal peaks were observed, corresponding to bound water, immobile water, and free water. As the concentration increased to 4% and 6%, a new relaxation peak appeared, representing monolayer water tightly bound to WPI or HS molecules through hydrogen bonds. The hydrogen proton binding ability of carrageenan was insufficient to bind monolayer water molecules, so the 2%, 4%, and 6% samples all showed three relaxation signal peaks. A significant decrease in T2 values and a gradual reduction in A25 were observed, indicating increased binding of hydrogen protons and reduced water migration rates.
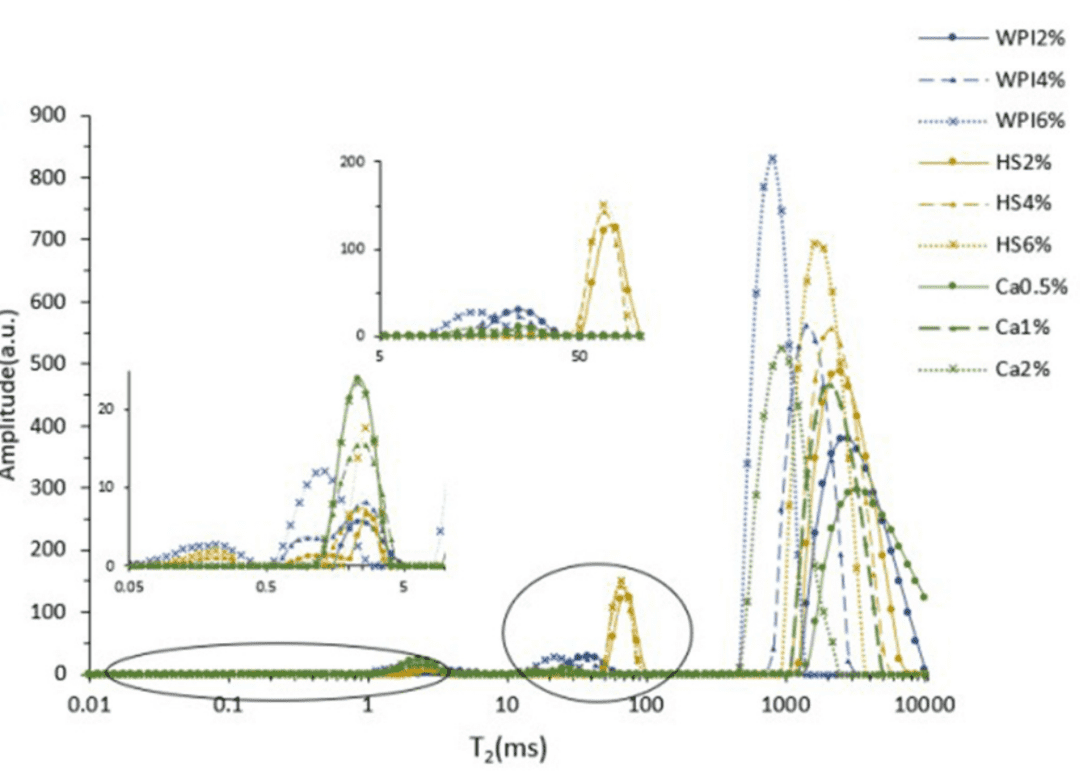
Fig. 2 T2 Relaxation Spectra of Samples with Different Concentrations
By analyzing the correlation between WPI, HS, and carrageenan suspension concentrations and relaxation results, as shown in Fig. 3, T25 represents free water hydrogen protons, and A25 represents total hydrogen protons. The concentrations of WPI, HS, and carrageenan show a linear relationship with T25 and A25.
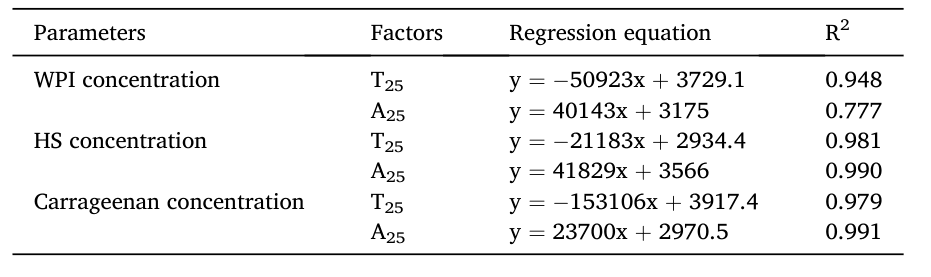
Fig. 3 Correlation Between WPI, HS, and Carrageenan Suspension Concentrations and LF-NMR Parameters
Emulsions Stabilized by Different Ratios of Emulsifiers:
Based on the decay curves of emulsions stabilized by different ratios of WPI, HS, and carrageenan, the maximum curvature time point (Tcur) and single-component relaxation time (T2W) were determined. Increasing the WPI content significantly accelerated the decay process of WPI-stabilized emulsions. When WPI concentration increased from 2% to 6% (W2%H0%C0%, W4%H0%C0%, and W6%H0%C0%), the T2W and Tcur values of the emulsions sharply decreased. Similarly, adding HS to the emulsion also shortened the decay process. This indicates that the presence of HS and carrageenan in WPI-stabilized emulsions can intervene in the state of hydrogen protons by altering WPI molecular interactions.
The T2 relaxation spectra of emulsions formed by WPI, HS, and carrageenan are shown in Fig. 4-1, 4-2, 4-3, and 4-4.
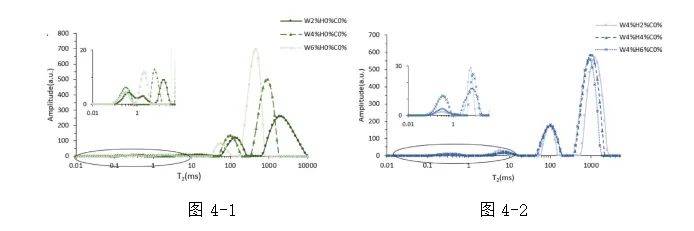

In the 2% WPI emulsion, besides the hydrogen proton groups from water molecules, the hydrogen protons from triglycerides in the emulsion also contributed to the T2 peaks. Compared to the three characteristic relaxation peaks of the 2% WPI dispersion, the 2% WPI-stabilized emulsion exhibited five peaks, T21-T25, corresponding to tightly bound water, weakly bound water, fixed water, oil, and free water. As WPI increased from 2% to 6%, the weakly bound water and fixed water peaks overlapped, and the oil signal peak decreased. This phenomenon indicates that the increase in WPI enhanced its binding with water molecules, providing more WPI-bound water molecules to cover oil droplets, thus reducing the migration rates of water and oil molecules.
The 2%–6% HS content (W4%H2%C0%, W4%H4%C0%, and W4%H6%C0%) emulsions, along with 4% WPI-stabilized emulsions (W4%H0%C0%), each showed four relaxation peaks. As the HS content increased, the areas of A24 and A25 gradually increased and decreased, respectively, indicating that HS promoted the migration of some free water molecules to form fixed water through hydrogen bonding, thereby enhancing the stability of the WPI-stabilized emulsion system by reducing the free water fraction in the aqueous phase.
After adding 0.5% and 1% carrageenan to the 4% WPI emulsion, four peaks appeared in the relaxation spectra (W4%H0%C0.5% and W4%H0%C1%). The T22, T24, and T25 peak values gradually shifted to lower T2 values, reflecting a decrease in water migration rates in the emulsion system, indicating that carrageenan had a stronger binding ability with hydrogen protons than WPI. Therefore, adding carrageenan can enhance the uniformity and stability of the emulsion system by restricting the overall migration rate of water molecules.
In the 1% carrageenan (W6%H0%C1%, W4%H2%C1%, W2%H4%C1%, W2%H4%C1%, and W0%H6%C1%) emulsions, samples with different WPI/HS ratios exhibited four peaks. As the WPI/HS ratio decreased, the T25 peak shifted to the right, and the area of the A25 peak gradually decreased, indicating an increase in free water migration rates and a continuous decrease in free water content. These results reflect the differences in WPI and HS in restricting water molecule migration, with HS being able to bind more water molecules than WPI.
Finally, the correlation between the concentrations of WPI, HS, and carrageenan in the emulsions and the relaxation results was analyzed, as shown in Fig. 5. In emulsions stabilized by WPI, HS, or carrageenan alone, there was a good correlation between the free water relaxation signal and the emulsifier concentration.
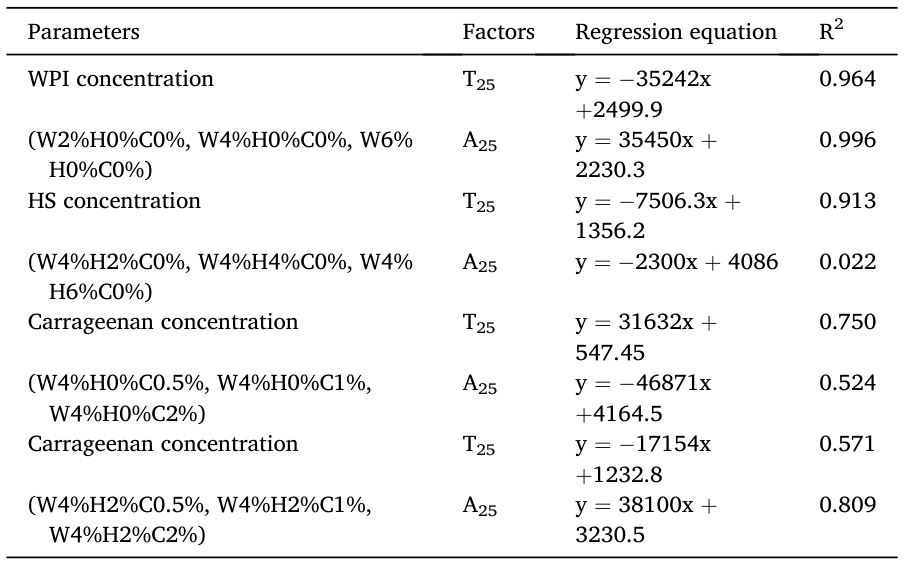
Fig. 5 Correlation Between Different WPI, HS, and Carrageenan Emulsions and LF-NMR Parameters
Conclusion:
The relaxation characterization of single-component emulsifier suspensions and emulsions stabilized by different ratios of emulsifiers using low-field nuclear magnetic resonance (LF-NMR) provides the following main conclusions:
1) Increasing the concentrations of WPI, HS, and Carrageenan decreases water mobility. Emulsifier concentration increases its binding capacity with hydrogen protons by affecting molecular interactions within or between molecules.
2) Interactions between WPI, HS, and Carrageenan. Increasing WPI concentration from 2% to 6%, or adding relatively low concentrations of HS and Carrageenan to WPI-stabilized emulsions, can enhance the stability of the WPI-stabilized emulsion system by restricting the movement of water molecules. HS is able to bind more water molecules than WPI.
3) In emulsions stabilized by WPI, HS, or Carrageenan alone, there is a good correlation between the free water relaxation signal and the emulsifier concentration.
References:
[1] Yinglin Zhong, Qingying Cai, Qingrong Huang, Xuanxuan Lu, Application of LF-NMR to characterize the roles of different emulsifiers in 3D printed emulsions, [J]. Food Hydrocolloids, Volume 133, 2022, 107993, ISSN 0268-005X.

Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top