Conventional methods for in-situ characterization of the early rapid hydration reactions of magnesium potassium phosphate cement (MKPC) are quite challenging. This study introduces the use of low-field nuclear magnetic resonance (LF-NMR) to monitor the reactivity of magnesium oxide in MKPC in-situ. The results are compared with the reactivity measured by XRD-Rietveld, BSE, and TG analysis methods. The results show that the deviation of the LF-NMR-measured reactivity from other methods is within ±20%, indicating that LF-NMR is a fast, effective, and accurate method for characterizing MKPC reaction kinetics.
Low-field nuclear magnetic resonance (LF-NMR) is an effective method for characterizing the pore structure of hardened cementitious materials.
The main principle of LF-NMR is to characterize the relaxation behavior of hydrogen protons in free water. Water molecules in pores of different diameters exhibit different longitudinal/transverse relaxation times and relaxation signal intensities. By monitoring the transformation of free water into chemically bound water, the hydration kinetics of cementitious materials can also be studied.
LF-NMR can continuously operate under non-destructive conditions, with each data point taking only 10-20 seconds to measure. Therefore, LF-NMR is suitable for the in-situ monitoring of rapid reaction kinetics of MKPC. However, to date, there have been no reports on quantitative calculation methods for the early reaction process of MKPC based on LF-NMR.
By monitoring the change in free water content using LF-NMR, the reaction kinetics of MKPC paste were measured in-situ. The accuracy of the LF-NMR-measured reactivity of MKPC was validated by comparing it with XRD-Rietveld, BSE, and TG analysis methods.
The LF-NMR analysis was conducted using the NMI20-015 V-I/15 mm analyzer provided by Suzhou Niumai Analytical Instrument Co., Ltd., with specific experimental parameters shown in Table 1.
Table 1 LF-NMR Experimental Parameters

Typical LF-NMR data and the related processing process are shown in Figure 1. The raw data (Figure 1a) is the transverse relaxation signal. By applying inverse transformation to the multiple exponents of the transverse relaxation signal, the T2 time distribution can be determined.
In the LF-NMR test, the protons in water molecules are initially excited to a high-energy state by external RF stimulation, and then return to the equilibrium state. The T2 relaxation time represents the recovery time. The small diameter of micropores facilitates energy exchange, and the T2 time is positively correlated with pore size, thus enabling analysis of microstructure features (Figure 1b).
The signal intensity/amplitude of LF-NMR is positively correlated with the amount of evaporative water. By characterizing the change in the amount of evaporative water in the sample, the reactivity of magnesium oxide can be calculated according to the reaction equation (1) (Figure 1c).
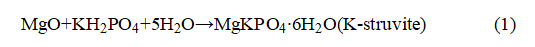
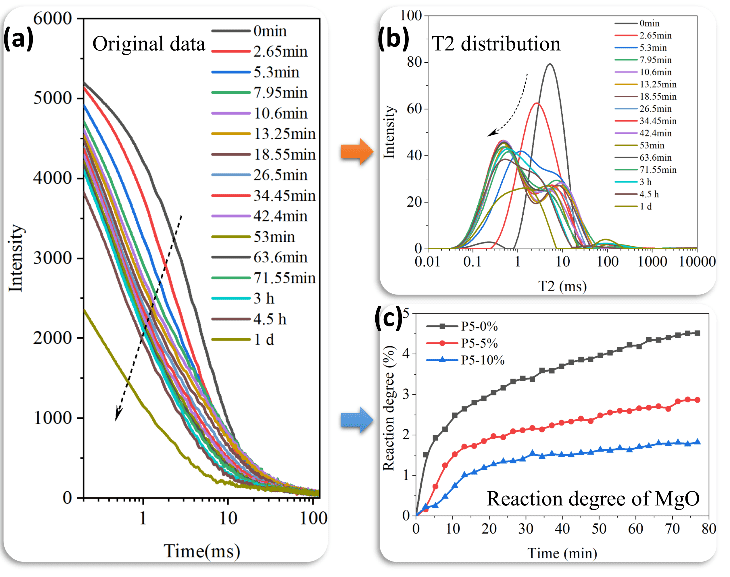
Figure 1 Typical LF-NMR Data Analysis Process: From Raw Data to T2 Time Distribution and Reactivity (a) Transverse Relaxation Signal; (b) T2 Distribution; (c) MgO Reactivity
The effect of M/P molar ratio, B/M mass ratio, and W/C ratio on the T2 distribution during the first 80 minutes of the reaction is shown in Figures 2–4.
Within the first 10 minutes, the main peak of the T2 distribution curve shifts from 10ms to approximately 1ms, indicating that the intense acid-base reaction in MKPC leads to rapid precipitation of hydration products, promoting pore structure refinement. As the M/P molar ratio increases (Figure 2) and the B/M mass ratio decreases (Figure 3), the main peak shifts earlier, suggesting that a high M/P ratio and low retarder content are favorable for MKPC reaction kinetics. Increasing the W/C ratio seems to have no effect on the main peak shift process, but due to the higher water content, the signal intensity increases (Figure 4).
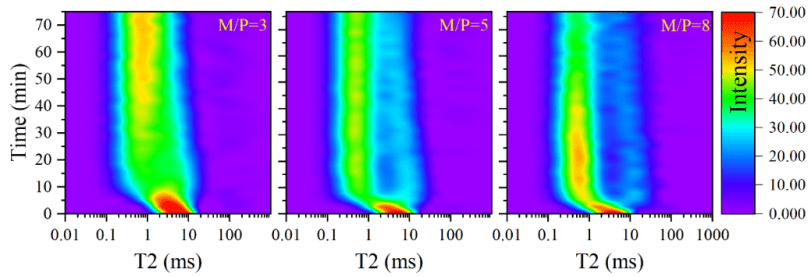
Figure 2 Effect of M/P Molar Ratio on T2 Distribution at the Initial Stage of Reaction
Figure 3 Effect of B/M Mass Ratio on T2 Distribution at the Initial Stage of Reaction

Figure 4 Effect of W/C Ratio on T2 Distribution at the Initial Stage of Reaction
The reactivity of magnesium oxide measured by in-situ LF-NMR is shown in Figure 5. As expected, the early reaction rate of magnesium oxide correlates positively with the initial M/P ratio within the first 3 hours (Figure 5a), indicating that the higher the magnesium oxide content, the more intense the acid-base reaction. Meanwhile, the reactivity of sample P5-5% after one day is higher than that of sample P8-5%, due to the insufficient amount of monopotassium phosphate, resulting in lower final reactivity of magnesium oxide at a higher M/P ratio.
As shown in Figure 5b, borax significantly inhibits the reaction of MKPC, and this inhibitory effect remains significant at 1 day. Increasing the W/C ratio promotes the hydration reaction of MKPC, which may be due to the higher water content in MKPC reactions and a larger space for the precipitation of hydrates. This phenomenon is also observed in the silicate cement system.
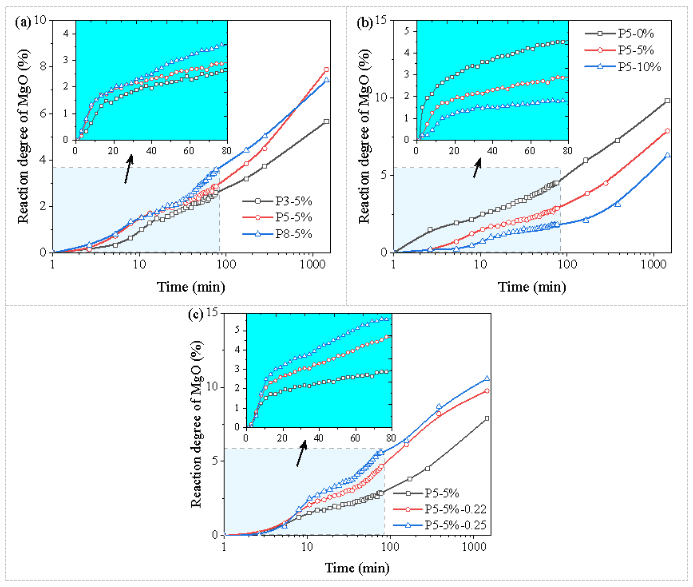
Figure 5 In-situ Characterization of MgO Reactivity: (a) at Different M/P Molar Ratios; (b) at Different B/M Mass Ratios; (c) at Different W/C Ratios
The results of the 1-day reactivity of magnesium oxide calculated from LF-NMR were compared with those determined by BSE-IA, XRD-Rietveld, and TGA methods, as shown in Figure 6.
The X-axis represents the reactivity determined by LF-NMR, and the Y-axis represents the reactivity determined by other methods. The reactivity of magnesium oxide determined by XRD-Rietveld is slightly higher than that determined by LF-NMR, while the TGA method gives lower values than LF-NMR.
As seen from Figure 6, the deviation between LF-NMR and other methods is within ±20%. Notably, the LF-NMR-measured reactivity of magnesium oxide is very close to the average value obtained from other methods, with a deviation within ±10%. These results suggest that LF-NMR provides accurate and feasible in-situ characterization of the early reaction kinetics of MKPC.
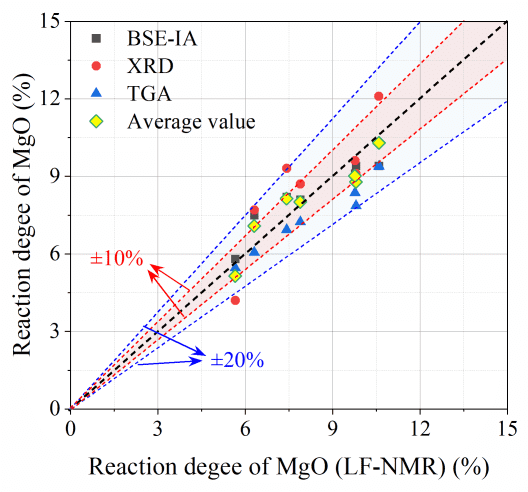
Figure 6 Comparison of MgO Reactivity Measured by Different Methods
[1] Ma S, Zhang Z, Liu X, et al. Quantitative characterization of the early hydration of magnesium potassium phosphate cement: In-situ experiment with low field NMR[J]. Construction and Building Materials, 2023: 131066.

Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top