This paper studies the presence of water in oil-based drilling fluids, particularly the interactions between water droplets and particles in “inverse emulsion drilling fluids.” Oil-based drilling fluids are typically considered a mixture of water-in-oil (W/O) emulsions and inorganic hydrophilic particles (such as barite and clay). However, the interaction between water droplets and inorganic hydrophilic particles has not been sufficiently considered. This study investigates the presence of water in the oil phase by adding hydrophilic particles BaSO₄ and hydrophobic particles PTFE to water-in-oil (W/O) emulsions.
The experimental results show that hydrophilic particles BaSO₄, compared to hydrophobic PTFE particles, can bind with water droplets, forming hydrated particle aggregates. Moreover, the actual presence of water in the oil-based drilling fluid was verified using 2D T₁-T₂ NMR technology. The study revealed that oil-based drilling fluid is not merely a W/O emulsion but a colloidal dispersion system of particles with bound water in the oil phase. This finding provides a scientific basis for designing the next generation of oil-based drilling fluids.
Drilling fluids play a crucial role in oil and gas drilling operations, serving key functions such as cooling and lubricating the drill bit, carrying rock cuttings to the surface, forming low-permeability filter cakes, maintaining wellbore stability, and preventing corrosion of the drill string. Typically, oil-based drilling fluids are regarded as a mixture of water-in-oil (W/O) emulsions and inorganic particles (such as barite and clay), with an oil-to-water ratio of approximately 8:2. However, this model neglects the interactions between water droplets and particles, which can significantly affect the performance of the drilling fluid.
In recent years, some studies have focused on the interactions between water droplets and hydrophilic particles, aiming to separate overly dispersed particles in the oil phase. For example, in the treatment of oil sand bitumen foam, finely dispersed particles wrapped in bitumen are stabilized in the oil phase, making particle removal difficult. By using hydrophilic polymers to enhance the hydrophilicity of bitumen-wrapped fine particles, followed by adding a small amount of water, the aggregation and settling of particles are promoted, allowing for particle removal from the oil phase. However, oil-based drilling fluids are typically considered a three-phase colloidal dispersion system, consisting of oil, water, and solid particles, where interactions between water droplets and particles are inevitable.
Therefore, this paper aims to explore the presence of water in oil-based drilling fluids, particularly the interactions between water droplets and hydrophilic particles. By adding hydrophilic BaSO₄ particles and hydrophobic PTFE particles to water-in-oil emulsions, this study investigates changes in the presence of water droplets in the oil phase and characterizes these changes using Low Field NMR (LF NMR) and other techniques. The results show that oil-based drilling fluids are not simple W/O emulsions but rather colloidal dispersion systems of particles with bound water in the oil phase.
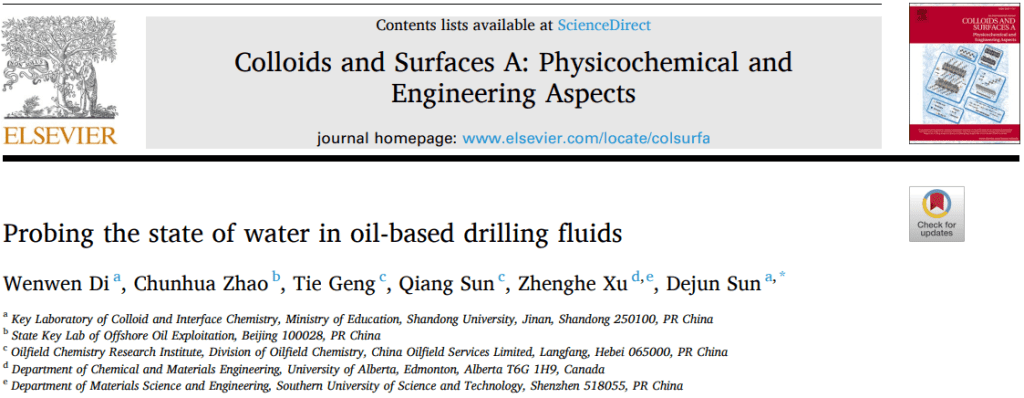
In the experiment, three systems were prepared: water-in-oil emulsion, followed by the addition of hydrophilic BaSO₄ particles and hydrophobic PTFE particles into the emulsion. The relaxation time distribution of water in the system was observed, and the changes in the presence of water droplets in the oil phase were explained by comparing the changes in water relaxation times before and after ultrasonic treatment. T2 relaxation time distribution: Before ultrasound (A) water-in-oil emulsion, after adding (B) hydrophilic BaSO₄ particles and (C) hydrophobic PTFE particles. (a–c) correspond to the systems after ultrasound. The particle volume fraction was 20%.
Low Field NMR (LF-NMR): Used to measure T2 relaxation times of hydrogen-containing samples to differentiate between different forms of water (e.g., free water droplets, bound water). By analyzing the distribution of T2 relaxation times, characteristic features of water phases in different states can be identified.
Figure 1: T2 distribution for different sample states
Figure 1 shows the presence of water in oil-based drilling fluid and its interactions with particles. Figures A–C show the T2 relaxation time for the system before and after ultrasonic treatment. Before ultrasound, the T2 relaxation time for the water-in-oil emulsion, after adding BaSO₄ and PTFE particles, was about 1629 ms, indicating that the water was in a relatively free state. After ultrasound, the T2 relaxation time for the water-in-oil emulsion remained long, indicating that although the water droplet size decreased, the hydrogen atoms in water remained in a relatively free state. For the BaSO₄ system, the T2 relaxation time decreased to 70 ms after ultrasound, indicating that the hydrogen atoms transitioned from a free to a bound state, which aligns with the phenomenon of water droplets binding to BaSO₄ particles. In contrast, no significant change in T2 relaxation time occurred in the PTFE system after ultrasound, showing a weaker interaction between water droplets and hydrophobic particles.
2D T1-T2 NMR: Further separates overlapping components, allowing for accurate identification of relaxation time characteristics for different hydrogen-containing components in complex systems.
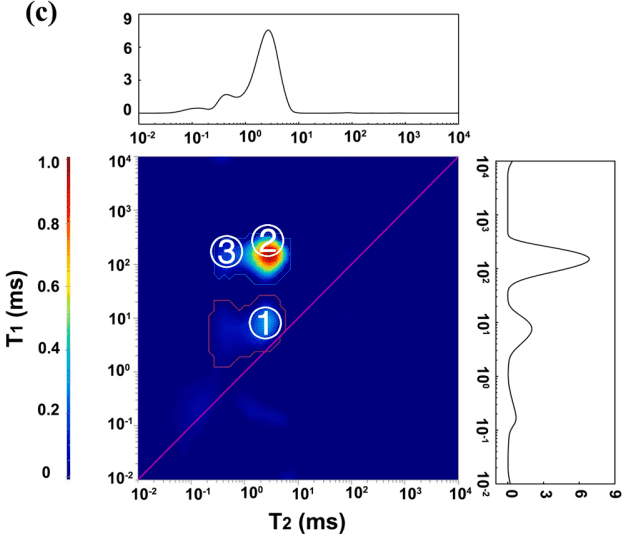
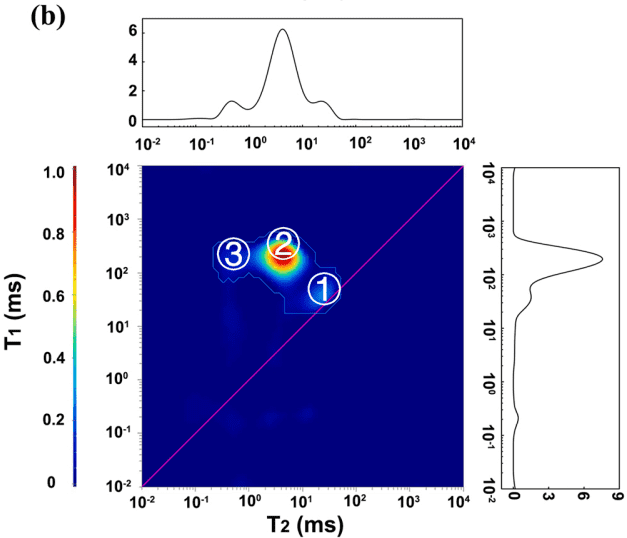

Figure 2: 2D T1-T2 plots of oil-based drilling fluid with 0-5-10% MnCl2
From Figure 2, it is evident that in actual oil-based drilling fluids, the T2 relaxation times for oil, saltwater, and organic components overlap. However, using 2D T1-T2 NMR measurements, three distinct regions can be separated, corresponding to different hydrogen-containing components. Region ① shows a shift in relaxation signals with the addition of MnCl2, indicating that this region represents water in the oil-based drilling fluid. The T2 relaxation time for region ① is 25 ms, much lower than the T2 relaxation time for water droplets, suggesting that the water is bound to particles in the oil-based drilling fluid.
This study, using nuclear magnetic resonance (NMR) technology, particularly LF-NMR and 2D T1-T2 NMR, in combination with LT-DSC and optical microscope observations, reveals changes in the presence of water in oil-based drilling fluids. The experimental results indicate that oil-based drilling fluids are not simple W/O emulsion systems but rather colloidal dispersion systems of particles with bound water in the oil phase. After adding hydrophilic particles, water molecules transition from free droplets to bound water, significantly altering their relaxation times and crystallization temperatures. These findings provide important insights into the microscopic structure of oil-based drilling fluids and optimization of their performance.
Newme has developed the NMR Drilling Fluid High-Temperature High-Pressure Simulation Evaluation System, based on the complex real environment in which drilling fluids circulate under high-temperature and high-pressure conditions downhole. This system simulates temperature and pressure conditions in drilling formations (0-150°C; 0-70 MPa), supporting long-duration experiments with varying temperature and pressure. It is used to characterize the distribution of oil and water components in drilling fluids under changing temperature and pressure; explain the mechanism of particle aggregation and settling; evaluate the effects of formulation improvements; and assess the aging stability of oil-based drilling fluids in complex, high-temperature, and high-pressure conditions using NMR technology.

Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top