
Calcium silicate hydrate (C-S-H) is the most important hydration product in Portland cement. In this study, a novel compaction method was used to prepare pure C-S-H samples. The compressive strength and flexural strength of C-S-H samples were tested. During the first drying-resaturation (D-R) cycle, the hydrolysis adsorption-absorption behavior of C-S-H was measured using thermogravimetric analysis and low-field nuclear magnetic resonance (LF-NMR). After the D-R cycle, a significant irreversible reduction in the gel pore volume was detected, while the interlayer pore volume remained unchanged. Based on the results, the gel pores were classified into collapsible gel pores (CGP > 2.6 nm), deformable gel pores (DGP, 1.4-2.6 nm), and rigid gel pores (RGP, < 1.4 nm). RGPs are stable in both size and volume, DGPs are stable in pore volume but undergo irreversible changes in pore size, while CGPs are fragile and tend to collapse, resulting in a reduction in pore size and volume after the first drying.
Drying shrinkage of viscous materials often leads to significant changes in the performance of the entire concrete structure. C-S-H is the most important hydration product in Portland cement, and the microstructural changes during its drying process are considered the main factors causing material drying shrinkage. These changes have been widely discussed based on several structural models of C-S-H. It has been noted that a large portion of the drying shrinkage during the first drying-resaturation (D-R) cycle is irreversible, and this irreversible shrinkage disappears in subsequent drying-resaturation cycles. During drying, the collapse of gel pores and the particle rearrangement at relative humidity (RH) levels above 11% are considered the main reasons for this irreversible shrinkage. Removal of interlayer water at low RH may form new chemical bonds, such as Si-O-Si between C-S-H layers, which is considered another cause of C-S-H shrinkage. On the other hand, the resaturation process is not a simple water injection process, as the pore structure gradually changes with increasing water content due to the expansion of interlayer spaces in C-S-H.
This study uses a novel method to prepare pure C-S-H samples. Low-field nuclear magnetic resonance (LF-NMR) is used to investigate the microstructural changes of C-S-H during the first D-R cycle, and a model is proposed to explain these changes.
Three different C-S-H samples were prepared by reacting CH and amorphous SiO2 powders at room temperature. The theoretical Ca/Si ratios were 0.9, 1.2, and 1.5. The water-to-solid ratio (W/S) was fixed at 0.8.
Two types of steel molds were used in this work: one was a cylindrical mold (ф20 mm), and the other was a rectangular mold (50 mm × 10 mm) for preparing C-S-H blocks. The structure of the molds is shown in the figure below. After the samples were extruded, they were removed from the molds and placed on shelves in a sealed chamber, where there was enough deionized water to fully saturate them. Three sets of C-S-H samples were prepared, labeled C09H, C12H, and C15H, with Ca/Si ratios of 0.9, 1.2, and 1.5 in the raw material formulation. The measured bulk density of the samples was 1.69 g/cm3. For the Ca/Si ratio of 0.9, another C-S-H sample was prepared, labeled C09L, with a measured bulk density of 1.50 g/cm3.
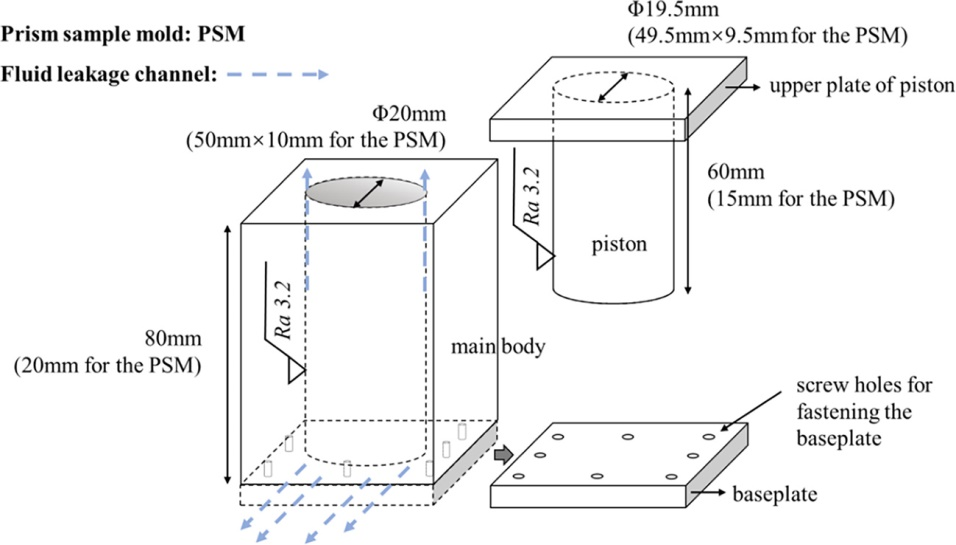
Figure 1 Schematic Diagram of Compaction Molds
1) Characteristics of Saturated C-S-H Samples:
After 6 months of curing under standard conditions, the block samples were directly used for mechanical testing without further treatment. The compressive strength of the cylindrical samples was tested using an electronic universal testing machine, with a loading rate set at 0.3 mm/min. The flexural strength of the cylindrical samples was tested using the three-point bending method.
For powder samples that required 29Si nuclear magnetic resonance spectroscopy and X-ray diffraction (XRD) testing, C-S-H blocks were quickly crushed and ground. The resulting powder was sieved and stored in sealed bags without any further drying treatment to maintain sample saturation.
2) Characteristics of C-S-H Samples During the First Drying-Resaturation Cycle:
C09H and C09L were used for the first drying-resaturation treatment. The powder samples were placed in a humidity-controlled space and subjected to the D-R cycle at 25°C.
The dynamic vapor sorption (DVS) method was used to achieve equilibrium at each RH change step. The low-field nuclear magnetic resonance equipment provided by Suzhou Newmai Analysis Instrument Co., Ltd. (magnetic field strength: 0.5T, magnetic field homogeneity <30ppm, system dead time = 13us, sequence: CPMG, TE = 0.1ms) was used to monitor the water distribution at different stages of the D-R cycle.
1) Characteristics of C-S-H Samples:
The results of XRD measurements are shown in Figure 2. It is clearly observed that C-S-H was successfully synthesized in each sample. This also implies that during the preparation process selected in this study, the Ca/Si ratio of the raw materials and compaction pressure did not significantly affect the crystalline structure of the obtained C-S-H samples. From the XRD, it is clear that the C09H and C09L samples have completely consumed CH, while for C12H and C15H, a significant amount of residual CH was detected even after 6 months of curing. This suggests that the C12H and C15H samples are a mixture of CH and C-S-H. Based on TGA data, the residual CH content in C12H and C15H was approximately 13% and 24% of the initial mixed CH content, respectively.
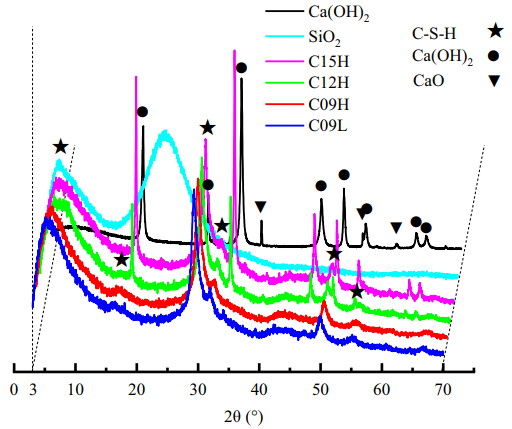
Figure 2 XRD Spectra of the Samples
As shown in Figure 3, silicon nuclear magnetic resonance was used to gather more information about the structure of the obtained C-S-H samples. For SiO2, two peaks were observed at -101.2 ppm (Q3) and -111.8 ppm (Q4). For C-S-H samples, two additional peaks were detected at -79.0 ppm (Q1) and -84.8 ppm (Q2), with the Q3 and Q4 signals disappearing, indicating the complete consumption of SiO2.
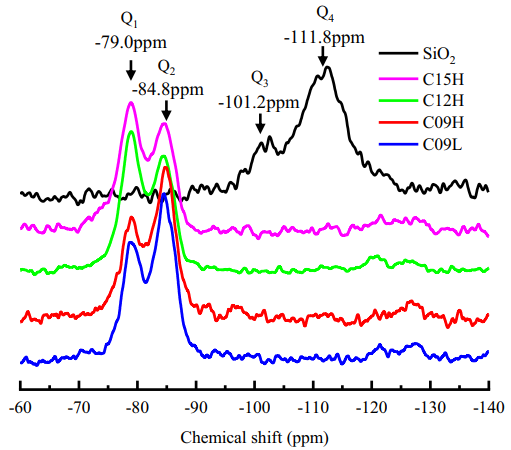
Figure 3 Chemical Shift Spectrum of the Samples
In summary, all C09 samples can be considered pure C-S-H, while the obtained C12H and C15H samples are essentially mixtures of C-S-H and CH, with the residual CH in C12H and C15H accounting for 8% and 16% of the balanced sample mass in RH, respectively.
2) Mechanical Properties of C-S-H Samples
Figure 4 shows the mechanical strength of C-S-H samples. It is clear that the compressive and flexural strengths of the C-S-H samples increase as the Ca/Si ratio decreases. Molecular dynamics simulations suggest that the decrease in the Ca/Si ratio of C-S-H leads to an increase in MCL, thereby improving the mechanical strength of C-S-H. It should also be noted that the small amount of residual crystalline CH in the samples (C15H and C12H) may act as an impurity and could be detrimental to the overall mechanical strength of the samples.
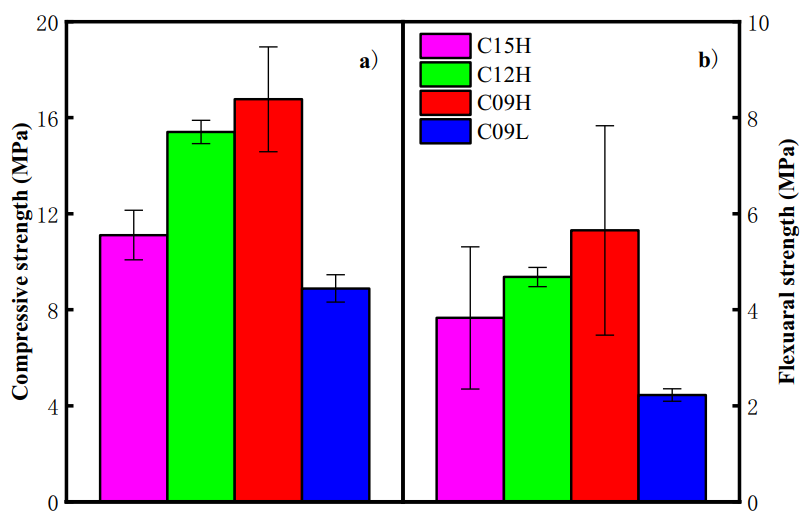
Figure 4 Mechanical Strength of the Samples: a) Compressive Strength; b) Flexural Strength
3) Reversible Microstructural Changes of C-S-H During the First D-R Cycle
To further understand the hydrolytic adsorption-absorption behavior and microstructural changes of C-S-H during the first D-R cycle, LF-NMR measurements were conducted. The LF-NMR results were inverted using the Butler-Reeds-Dawson (BRD) algorithm. Typical results are shown in Figure 5. All measured samples exhibited two peaks at T2 values of about 0.06 ms and 0.2 ms, which represent interlayer water and gel pore water in C-S-H structure.
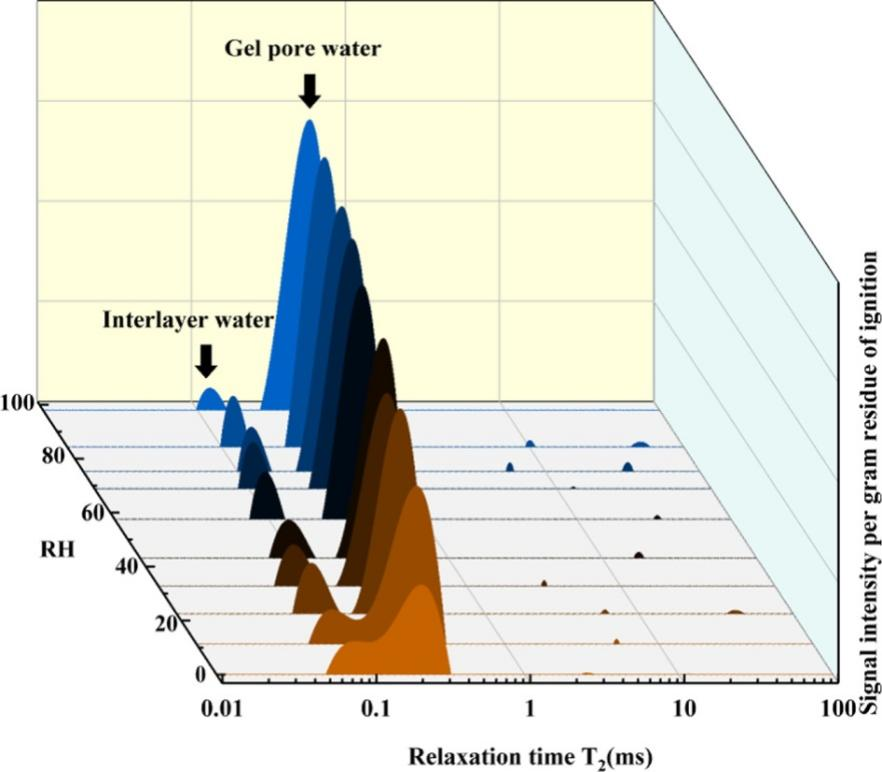
Figure 5 T2 Spectrum of C09H during the Cycle
In this study, for C09L and C09H, the content of interlayer water did not show significant changes during the first D-R cycle. In other words, the interlayer water was sufficiently stable to withstand drying even at RH levels below 11%.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top