This study primarily focuses on further optimizing the performance of solid rocket motor (SRM) casings under increasingly stringent aerospace technology requirements. The research used the co-curing technique to prepare carbon fiber reinforced polymer (CFRP) and ethylene propylene diene monomer (EPDM) rubber composites. By varying the temperature, heating time, and the type of curing agent, the properties of the CFRP/EPDM composite were adjusted to achieve the best manufacturing process. The crosslinking density tested by nuclear magnetic resonance (NMR) was 3.459 × 10-4 mol/cm3, with a 90° peel strength of 2.342 N/mm and interlaminar shear strength (ILSS) of 82.08 MPa. These results indicate that heating for 20 minutes at 160°C using a DCP/S vulcanization system yields the best mechanical properties of the composite.

CFRP and EPDM rubber, due to their excellent performance characteristics, are key components of SRMs. CFRP is used as the SRM casing due to its low density, high strength, dimensional stability, and corrosion resistance. EPDM rubber, with its density (0.85 g/cm3), low thermal conductivity, and high thermal stability, serves as the thermal barrier for SRMs. However, during rocket launches, high-speed heat flux can generate extreme temperatures (2000-4000°C) and pressures (approximately 60 bar), which can cause degradation and delamination of the barriers. Therefore, the interface bond strength between CFRP and EPDM rubber must be sufficiently strong to withstand the impact of high-speed heat flux.
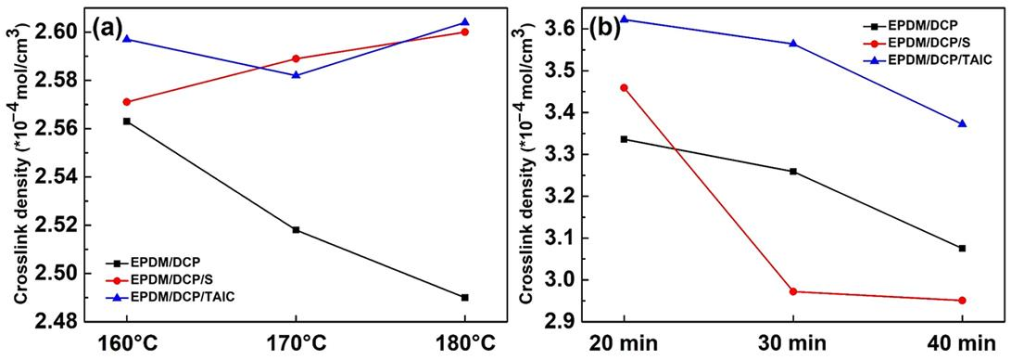
Figure 2
The effect of temperature, time, and curing agent type on the crosslinking density of EPDM rubber is shown in Figure 2. As shown in Figure 2a, as the temperature gradually increases, the crosslinking density of EPDM/DCP rubber decreases, while the crosslinking densities of EPDM/DCP/S and EPDM/DCP/TAIC remain at relatively reasonable values. One possible explanation is that the presence of curing auxiliaries like S and TAIC can introduce C-S or C-O bonds into the crosslinking system, thereby improving the integrity of the crosslinked structure. Additionally, under prolonged high-temperature conditions, C-S, C-C, and C-O bonds in EPDM rubber break, leading to a decrease in its crosslinking density. Therefore, the optimal curing temperature for EPDM rubber is 160°C. With an increase in time, the crosslinking density of EPDM rubber shows a continuous decreasing trend, with EPDM/DCP/S showing the fastest decrease (Figure 2b). The bond energies of C-S, C-C, and C-O are 276 kJ/mol, 334 kJ/mol, and 364 kJ/mol, respectively, with the C-S bond having the lowest bond energy. Under prolonged high-temperature conditions, the C-S bond is more easily broken than the others. The optimal curing time for EPDM rubber is 20 minutes. At 160°C for 20 minutes, the crosslinking densities of EPDM rubber reach 3.336, 3.459, and 3.622 × 10-4 mol/cm3, respectively. The crosslinking densities of EPDM/DCP/S and EPDM/DCP/TAIC are significantly higher than that of EPDM/DCP, indicating that the incorporation of S and TAIC promotes a higher crosslinking density of the rubber.
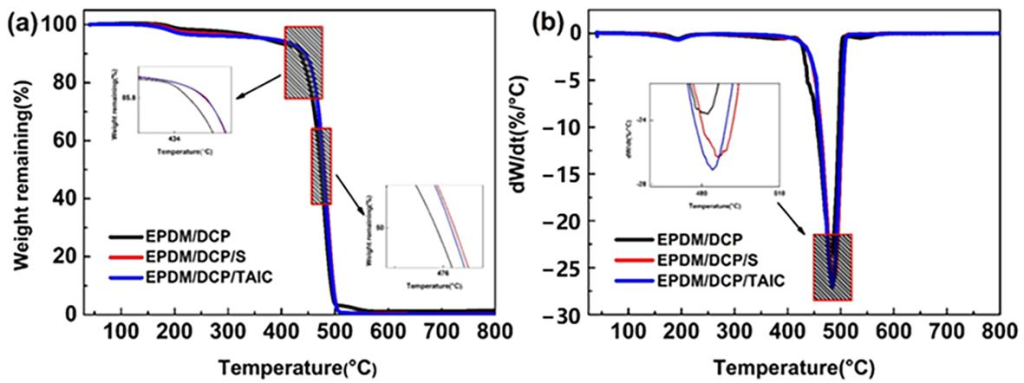
The thermogravimetric (TG) and differential thermogravimetric (DTG) curves of the pyrolysis reaction of EPDM rubber are shown in Figures 9a and 9b. Compared with EPDM/DCP, Ti, EPDM/DCP/S, and EPDM/DCP/TAIC, both the Tp and Tr of EPDM/DCP/S and EPDM/DCP/TAIC significantly increase, but their contents increase significantly, and the carbonization yields of EPDM/DCP/S and EPDM/DCP/TAIC slightly decrease. This shows that EPDM/DCP/S and EPDM/DCP/TAIC have higher thermal stability than EPDM/DCP. This can be attributed to the combined effect of S and TAIC, which increases the crosslinking degree of EPDM rubber, thereby enhancing its thermal performance and stability.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top