Test squid slices at different drying stages to observe moisture migration during the drying process.
2.1 Materials
Seven samples dried with the same method but for different durations: T5-1h, T5-2h, T5-3h, T5-4.5h, T5-5.5h, T5-7h, and T5-8.5h. (T5 indicates the drying method; “1h” indicates a drying time of 1 hour.)
2.2 Instrumentation
NMI20 low-field NMR imaging analyzer with a 15 mm probe coil diameter.

2.3 Sample Preparation
Cut each sample into cubes of approximately 2–3 mm per side and place them into a 15 mm OD test tube; target packing height: 15–20 mm.
2.4 Experimental Parameters
Data acquired using the CPMG sequence.
(i) Background
The state of water—its binding and mobility—is critical in foods, directly affecting rheology and stability. Low-field NMR has been widely used to study bound water in biological systems by measuring the transverse relaxation time T2 of hydrogen nuclei, which reflects molecular mobility. T2 shortens when water is tightly bound to substrates (restricted mobility), while free water shows longer T2. Thus, LF-NMR reveals the strength and extent of water binding.
(ii) Test Results
(1) Moisture migration (T2 spectra)
With one drying method and varying times, post-dried samples can be grouped into two stages:
Stage 1: Samples T5-1h to T5-3h. Common features: muscle structure intact; gel-like appearance; white and semi-transparent.
Stage 2: Samples T5-4.5h to T5-8.5h. Common features: muscle structure disrupted; dry texture; yellowish; reduced transparency.
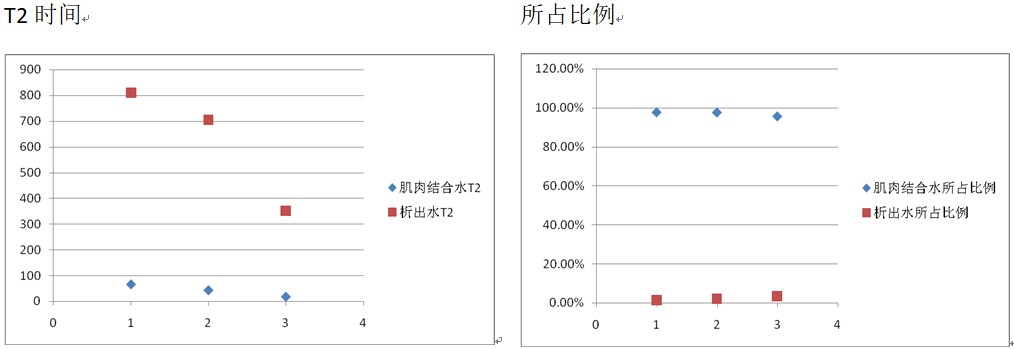
Relaxation analysis — Stage 1

The Stage 1 T2 spectra show two prominent components: a larger early peak and a smaller later peak. Both shift to shorter T2 with longer drying. The larger peak’s fraction decreases over time, while the smaller peak’s fraction increases. As drying proceeds, squid muscle contracts, water-holding capacity declines, and water is expelled—yet water that remains becomes more tightly bound. Hence, the larger peak reflects muscle–water binding: its T2 shortens (stronger binding) and its fraction drops (less retained water). The smaller peak reflects expelled water: its T2 also shortens (expulsion becomes harder), while its fraction increases (bound water converting to free water).
Relaxation analysis — Transition
By inspection, T5-3h appears gel-like, while T5-4.5h is dry. The former indicates intact muscle; the latter indicates structural damage. A sharp change in water binding is therefore expected across this interval.
As shown, the expelled-water and muscle-bound-water peaks present at 3 h disappear by 4.5 h. Even the shortest expelled-water T2 exceeds the initial muscle-bound-water T2; yet beyond ~65 ms no peaks remain at 4.5 h. Once no further water is expelled, the muscle has lost water-holding capacity—its bound-water signature vanishes. From Stage 2 onward, the spectra no longer represent expelled water vs. muscle-bound water.
Relaxation analysis — Stage 2
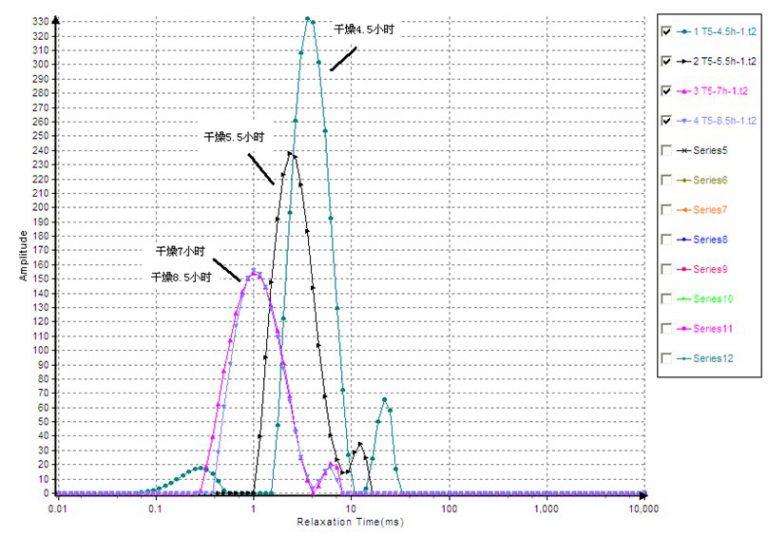
Following the transition, muscle structure is compromised and cannot retain water. Continued moisture loss in Stage 2 therefore relates to components binding water more tightly than the original muscle matrix—likely proteins. The observed doublet resembles spectra from cross-linked elastomers, suggesting signals from hydrogens on protein backbones. Crosslink theory assigns the shorter T2 to crosslink sites and the longer T2 to dangling chains; higher crosslink density shortens both T2 values, increasing the short-T2 fraction while decreasing the long-T2 fraction. Here, with longer drying, both T2 components decrease; the short-T2 fraction rises while the long-T2 fraction falls—consistent with denaturation and densification of a protein network and increasing sample rigidity.
1) With longer drying, muscle-bound water in squid progressively expels (Stage 1).
2) As drying continues, proteins denature and the texture hardens (Stage 2).
3) A critical point of complete muscle destruction and loss of water-holding capacity occurs between 3 h and 4.5 h.
4) Protein denaturation begins between 4.5 h and 5.5 h.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top