Chocolate is a high-calorie food that is loved by many, characterised by relatively low protein content and high fat content. While chocolate offers certain health benefits, its high calorie content can contribute to weight gain if consumed excessively.
Fat content is an important physicochemical indicator for evaluating chocolate quality. Currently, there are no strict standardised methods for measuring fat in chocolate. Different testing methods can yield varying results. Common approaches include Soxhlet extraction, acid hydrolysis, and low-field Nuclear Magnetic Resonance (NMR). Long-term experimental evidence suggests that both Soxhlet extraction and acid hydrolysis may not provide results that accurately reflect the true fat content of chocolate products.

The Soxhlet method primarily uses organic solvents such as ether or petroleum ether to extract fat. After evaporating the solvent, the remaining material—known as crude fat—may also contain pigments, volatile oils, waxes, and other compounds. This method is unsuitable for high-sugar foods because sugars can dissolve into the solvent and be collected in the receiving flask, leading to inflated fat values. Chocolate, being high in sugar, often yields results that deviate from the actual fat content. Another major factor is the presence of milk powder in chocolate; milk fat cannot be fully dissolved in ether, further contributing to measurement errors.
Acid hydrolysis is suitable for processed foods, clumped insoluble samples, and samples with water content that is difficult to remove. Strong acids are used to break down proteins and cellulose, freeing fats for extraction with ether. In chocolate, strong acids can disrupt milk fat globule membranes, releasing milk fat and allowing measurement of total fat. However, high sugar content in chocolate can still affect results. Additionally, human factors in the process—such as incomplete or inaccurate collection of the ether layer—can introduce slight deviations after ether evaporation, impacting measurement accuracy.
Low-field NMR measures fat content based on the proportional relationship between the NMR signal intensity and the fat present in chocolate. By calibrating the NMR signal per gram of sample against known fat content, the fat content of unknown samples can be quantified accurately.
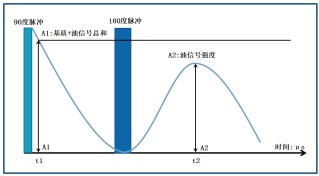
Measurements are conducted using a spin-echo sequence. The first figure illustrates the spin-echo sequence and corresponding NMR signals. After a 90° RF pulse, the Free Induction Decay (FID) signal is measured at time t1. The amplitude (A1) corresponds to the number of hydrogen nuclei in both solid and liquid phases (matrix and fat) of the sample. Following a 180° pulse, the spin-echo amplitude (A2) is recorded, at which point the solid-phase signal has decayed to zero, leaving only the fat signal. A2 is proportional to the fat content, allowing precise quantification.
After calibration using 3–6 samples of known fat content, unknown samples can be tested in 30 seconds to 3 minutes. The process is fast, non-destructive, and suitable for real-time industrial online testing.

Recommended Instrument: PQ001 Series Low-Field NMR Analyser
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top