Polymeric Compounds, abbreviated as polymers, also known as macromolecular compounds, generally refer to compounds with molecular weights ranging from thousands to millions. The majority of polymeric compounds are mixtures of homologues with different molecular weights, so the molecular weight of a polymeric compound is the average molecular weight. These compounds are made up of hundreds or thousands of atoms connected by covalent bonds. Despite their large molecular weights, they are formed by simple structural units connected in a repeating manner.
The molecular structures of polymers can be classified into two basic types: The first type is the linear structure, and polymeric compounds with this structure are called linear polymers. The second type is the branched structure, and polymers with this structure are referred to as branched polymers. In addition, some polymers have branches and are considered linear polymers. Some polymers, although having cross-linked molecular chains, have minimal cross-linking, and such structures are known as network structures, which belong to the branched structure category.
Entanglement is one of the key characteristics of polymeric compounds, influencing many physical properties such as viscosity and rheology. Therefore, the entanglement phenomenon of polymers is widely recognized. According to recent research, polymer chain entanglement can be divided into topological entanglements and entropic entanglements, but the mechanism of entanglement between chains is not yet fully understood. Qualitatively, the degree of entanglement in a solution depends on factors such as polymer molecular weight, solution concentration, and temperature. Entanglement directly affects the movement of polymer molecules, and therefore NMR relaxation spectra, which are effective tools for studying molecular motion, can also be used to study polymer entanglement.
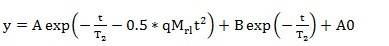
The dipolar interaction between hydrogen protons within and between polymer molecules causes nuclear magnetic resonance (NMR) transverse relaxation. When the temperature is far above the polymer’s glass transition temperature, this dipolar interaction in the polymer network is considered the average of thermal molecular motion. Since hydrogen protons in the polymer’s single chain serve as probes for NMR measurement, a modified single-chain model is introduced to explain the transverse relaxation of polymers. This model (referred to as the XLD model below) has been successfully tested and described in several publications.

Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top