Hydrogels are polymer materials with vast application potential. However, most hydrogels contain high water content, which limits their usability across wide temperature ranges. Below freezing temperatures, significant changes in physical and chemical properties occur—ice crystal formation disrupts the polymer network, turning the gel brittle and reducing its transparency. This dramatically restricts its functionality. Developing hydrogels with anti-freezing capability and reliable low-temperature mechanical performance is a promising research direction.
In hydrogels, water molecules are generally classified as bound water or free water, based on their interaction with the polymer network. Free water is primarily responsible for freezing behavior at around –10 °C. To suppress freezing, cryoprotectants such as inorganic salts or organic solvents are often added to reduce the freezing point of the free water component.

Currently, conventional mechanical testing is used to evaluate the anti-freezing performance of hydrogels. These methods tend to be time-consuming and destructive. In contrast, the VTMR20-010V-I variable temperature LF-NMR analyzer (by Suzhou Niumag Analytical Instrument Corp.) provides a fast, in-situ, and non-destructive solution by assessing the state of water molecules within the gel to determine its anti-freezing properties.
Three hydrogel samples (a, b, c) were tested, each with increasing salt content. Their relaxation behaviors were analyzed under temperatures of 25 °C, 0 °C, –10 °C, –20 °C, and –30 °C.
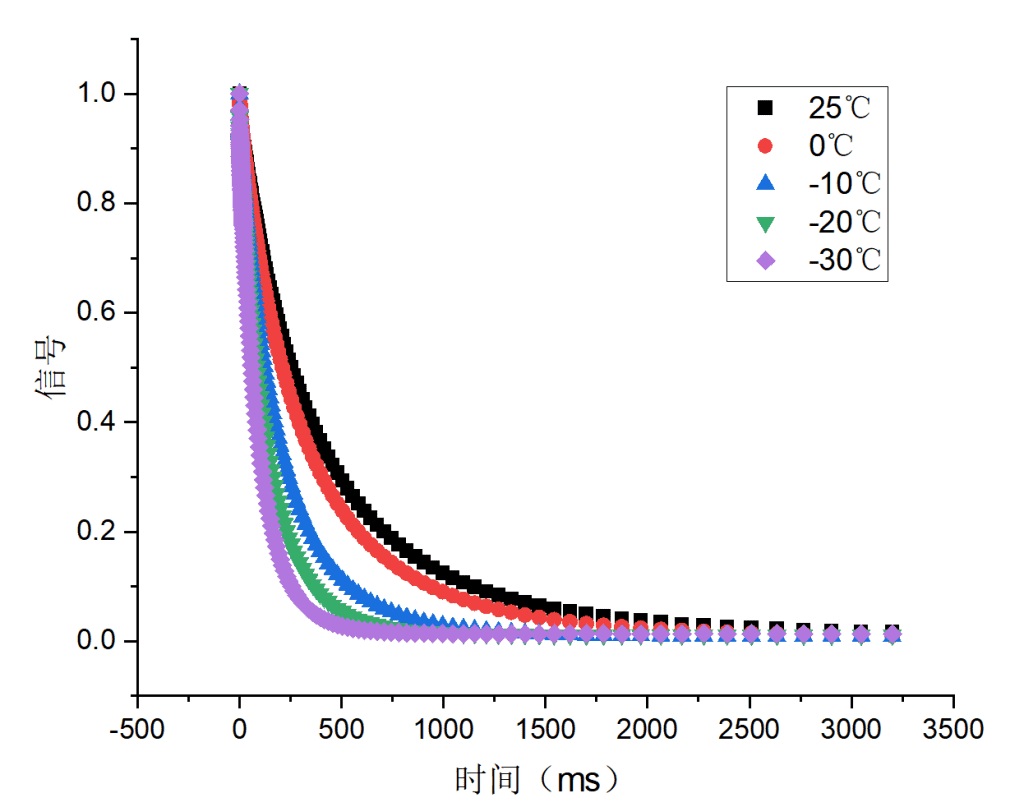
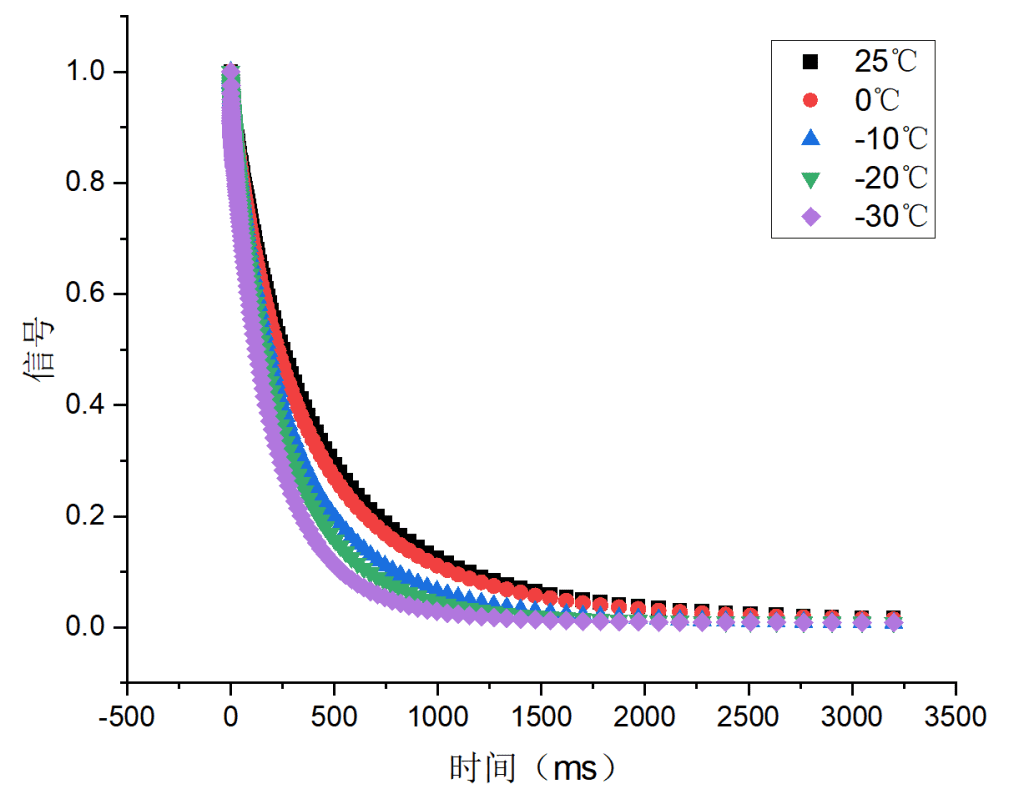
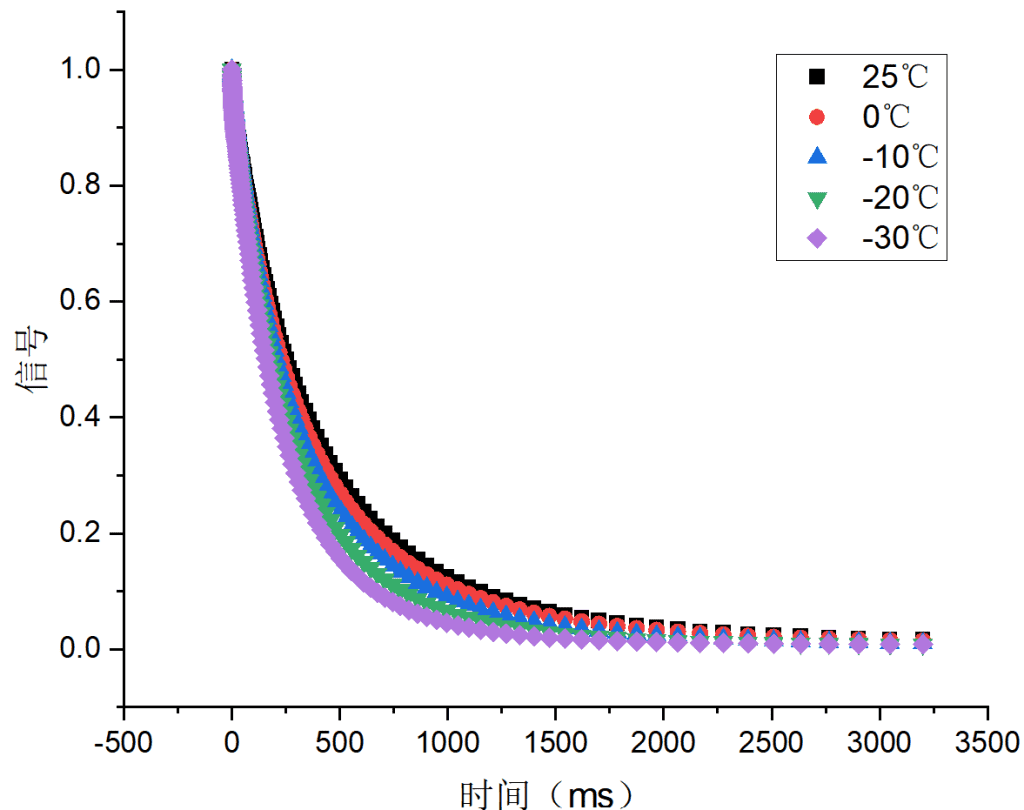
Figure 1: Relaxation behavior of samples a, b, and c
All three samples showed similar relaxation trends. As temperature decreased, the mobility of free water was reduced, causing faster relaxation. Among them, sample c exhibited the smallest changes, indicating that higher salt content limited the temperature sensitivity of free water. This suggests sample c has superior anti-freezing properties compared to a and b.
Further inversion of the relaxation curves provided insights into the proportions of free water, intermediate water, and bound water in each gel sample.
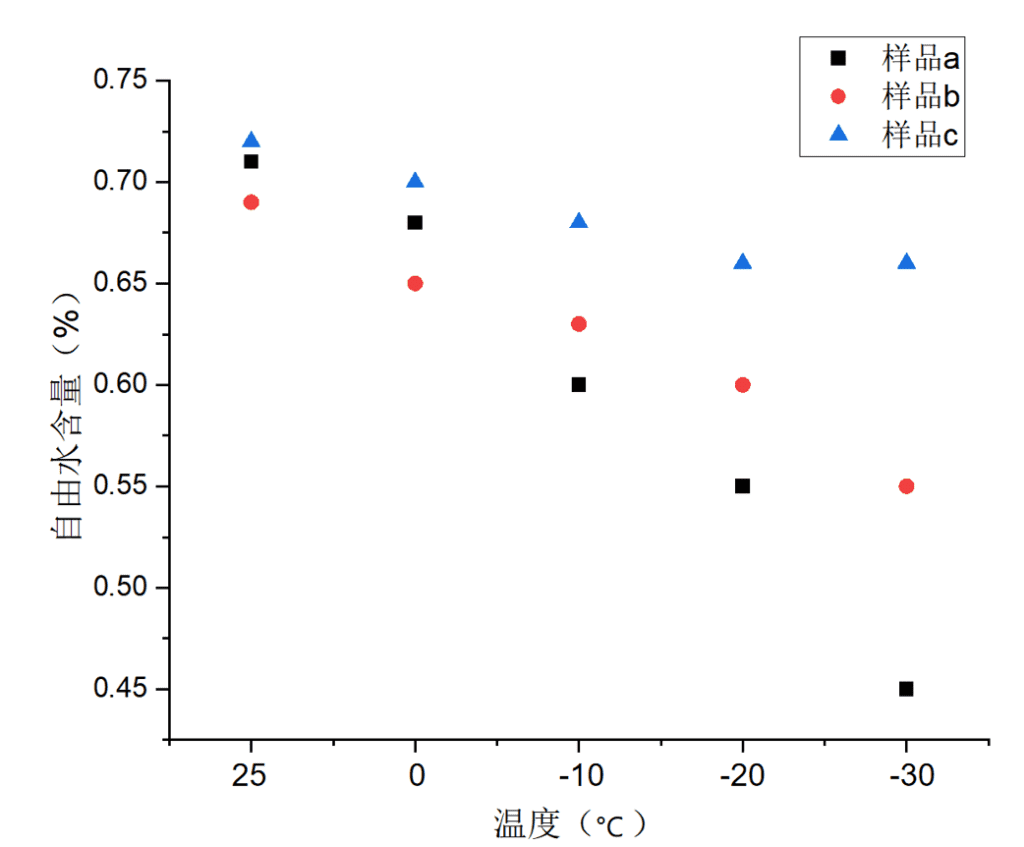
Changes in free water proportion determine the hydrogel’s ability to resist freezing. The smaller the change across different temperatures, the stronger the freeze resistance. According to the graph, sample c showed the least fluctuation in free water content, further confirming its enhanced anti-freezing performance relative to samples a and b.
If you’re interested in this application, feel free to contact us: 15618037925
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top