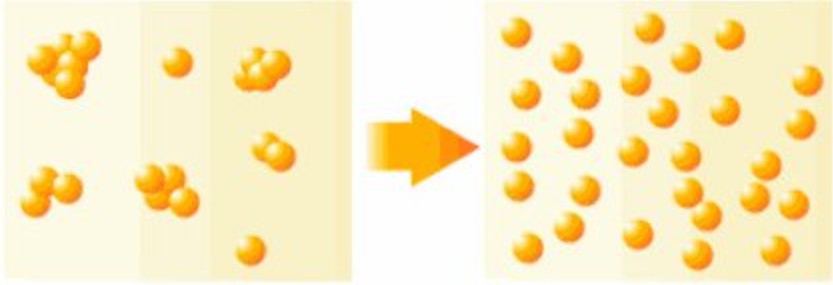
Agglomerates: Refers to primary particles joined face-to-face. Their total surface area is significantly smaller than the sum of the individual particles, making them very difficult to redisperse.
Aggregates: Refers to primary particles connected by point or edge contacts, or smaller particles attached to the surfaces of larger ones. Their total surface area is larger than that of agglomerates but still smaller than the total area of unbound particles. These are easier to redisperse. Both agglomerates and aggregates are also referred to as secondary particles.
Flocs: Refers to loose, fluffy structures that form to reduce surface energy when a system’s surface area increases. Typically, this occurs due to bridging effects of macromolecular surfactants or water-soluble polymers, binding particles together in soft, cotton-like structures. The distance between ions in these structures is much greater than in agglomerates or aggregates.
Surface wettability plays a critical role in powder dispersion. It forms the theoretical foundation for processes such as powder dispersion, solid–liquid separation, surface modification, and granulation. The wetting of solid particles by liquids primarily depends on the surface characteristics of the particles themselves. This is governed by the chemical composition and microstructure of the surface. Solids with higher surface free energy are more easily wetted, whereas low surface energy reduces wettability. Therefore, engineering solid surfaces with high surface free energy is essential for creating superhydrophilic or superhydrophobic surfaces.
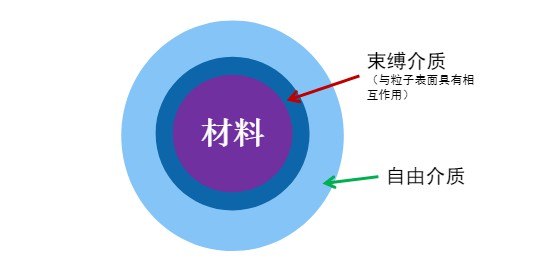
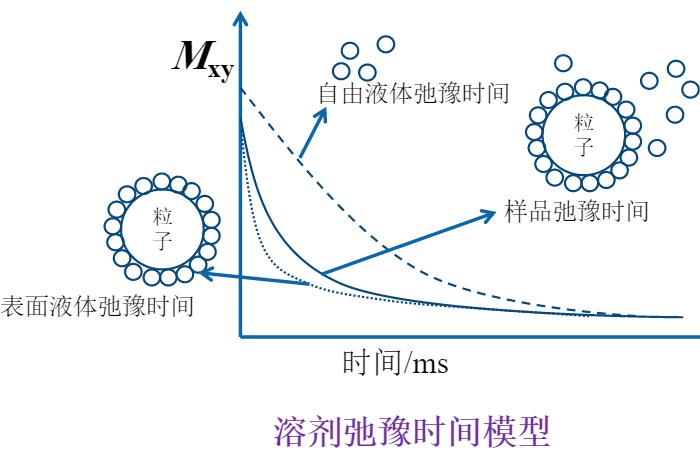
In a wetted particle system, liquid molecules tend to adhere to particle surfaces, where their motion becomes restricted due to adsorption by the inorganic phase. In contrast, molecules not in contact with particles remain in a free state. The NMR relaxation time of a liquid molecule is closely related to its mobility—freely moving molecules have significantly longer relaxation times than those in bound states. Better-dispersed systems generally adsorb more solvent, resulting in shorter average relaxation times. As such, low-field nuclear magnetic resonance (LF-NMR) can be used to measure the relaxation time of suspensions and calculate the wettable specific surface area (effective adsorption area) of particles. This provides insight into particle agglomeration behavior, dispersion stability, surface affinity, and wettability characteristics.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top