Alkali-Activated Slag (AAS) cement is a hydraulic cementitious material composed mainly of dried granulated blast furnace slag (GBFS) or other latent hydraulic materials (e.g., fly ash), combined with a moderate amount of Portland cement clinker, a small quantity of gypsum (CaSO₄·2H₂O), and an appropriate dosage of alkaline activators. In typical formulations, slag accounts for 80–85% of the total weight, alkali activators 5–10%, and the remainder is clinker. This green and eco-friendly binder not only offers superior mechanical and durability properties but also comes at a relatively low cost, making it one of the most promising and widely researched sustainable construction materials today.
Key Concept
This hydraulic binder is produced by blending dried granulated blast furnace slag (or other latent hydraulic materials like fly ash) with a suitable amount of Portland cement clinker, a small quantity of gypsum, and alkali activators. Slag comprises 80–85% of the total, alkali activators 5–10%, and the rest is cement clinker. AAS-based materials are energy-efficient, environmentally friendly, and possess excellent strength and durability. They offer a cost-effective alternative to traditional cement, making them a research hotspot in sustainable construction materials.
Experimental Method
01. Experimental Materials
The primary materials used in the experiment include amorphous blast furnace slag from a steel manufacturer and P.I 42.5 Portland cement. X-ray fluorescence (XRF) analysis was employed to determine the chemical compositions of both slag and cement, as shown in the table below.
A laser particle size analyzer was used to determine the particle size distribution of the selected materials, as shown in the figure below.

Figure 1. Particle size distribution curve of cement and alkali-activated slag
Additionally, river sand with a maximum particle size of 2.36 mm, fineness modulus of 2.75, and bulk density of 2530 kg/m³ was used to prepare the mortar specimens. Industrial-grade granular NaOH (purity: 99 ± 1%) and sodium silicate solution (containing 8.3% Na₂O, 26.5% SiO₂, and 65.2% H₂O) were selected as alkali activators. Alkaline solutions with SiO₂/Na₂O molar ratios (modulus) of 0, 0.5, 1.0, and 1.5 were prepared 24 hours prior to the experiment.
02. Mix Design and Specimen Preparation
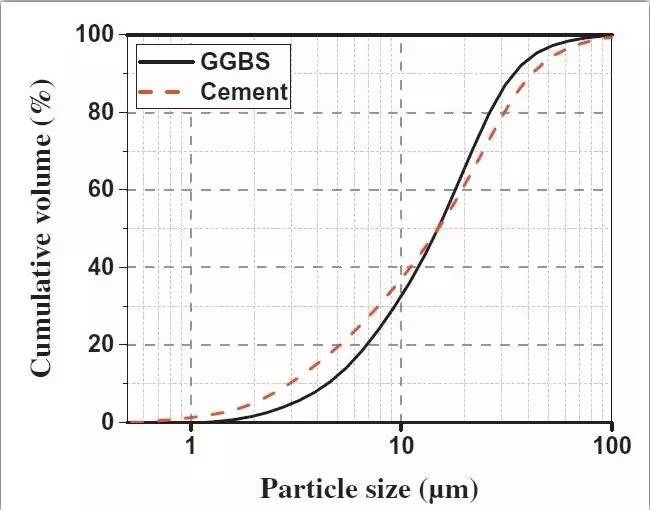
Figure 2. Grouping of AAS and cement mortar mix proportions
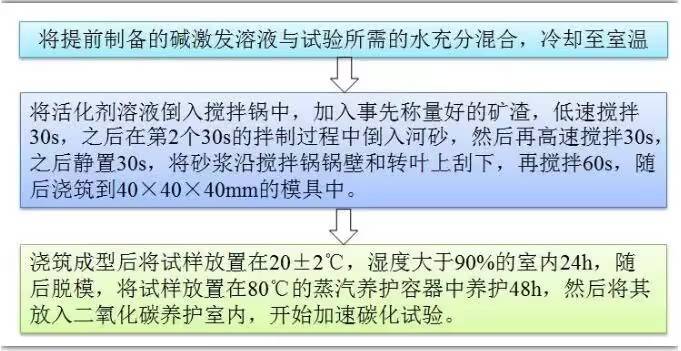
Figure 3. Specimen preparation process flowchart
03. Micropore Structure Testing
To investigate the microstructural changes before and after carbonation in PC and AAS mortars, low-field nuclear magnetic resonance (LF-NMR) testing was conducted using the MacroMR12-150H-I system from Niumag. Cubic mortar specimens (40×40×40 mm) were dried in an oven at 60°C for 24 hours, cooled to room temperature (20°C), and then vacuum saturated before NMR testing.
Experimental Results
01. Effect of Carbonation on Compressive Strength
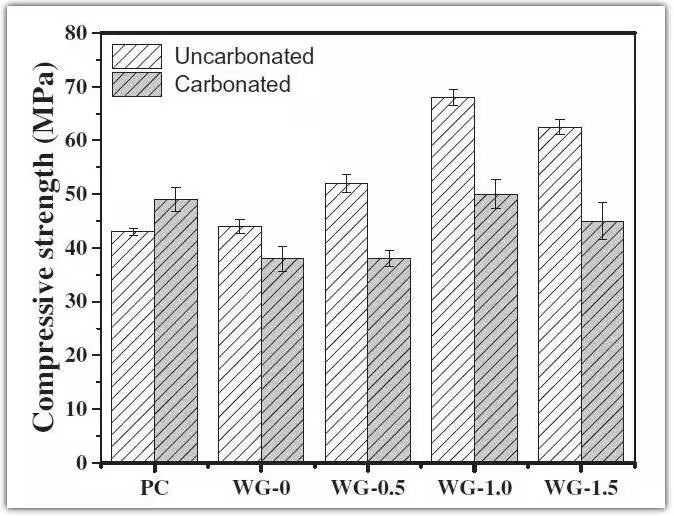
Figure 4. Compressive strength of AAS and PC mortar before and after carbonation
As shown in the figure, carbonation slightly improves the compressive strength of PC mortars. This is mainly due to secondary reactions between carbonation products and hydration products, forming fine crystals that fill internal pores, densify the matrix, and enhance strength.
In contrast, carbonation reduces compressive strength in AAS mortars at all concentrations. This is attributed to two primary factors: (1) the slow crystallization rate of calcium carbonate, especially in low Ca/Si C-S-H gels, which suppresses CaCO₃ formation in alkali-activated systems; (2) although low Ca/Si C-S-H gels exhibit high durability, once decalcified, their structure rapidly degrades, reducing cohesion within the matrix.
02. Effect of Carbonation on Porosity
This study examined the influence of carbonation on porosity, dry-state NMR signal, and volumetric change in AAS and PC samples using LF-NMR. Results are shown in Table 3 below.
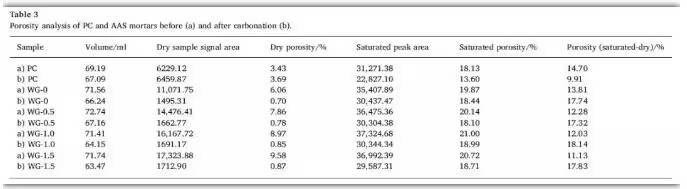
Significant differences were observed between AAS and PC in terms of volume change, NMR signal, and porosity before and after carbonation. All samples exhibited shrinkage post-carbonation, but PC mortars had lower shrinkage. This is likely due to the transformation of Ca(OH)₂ into CaCO₃, which has been shown to increase volume by 11.8%. Additionally, higher sodium silicate modulus increased shrinkage: by 7.43%, 7.67%, 9.98%, and 11.53% for modulus values of 0, 0.5, 1.0, and 1.5 respectively. This may be due to delayed CaCO₃ crystallization and interference from hydrotalcite formation during carbonation.
03. Effect of Carbonation on Pore Structure
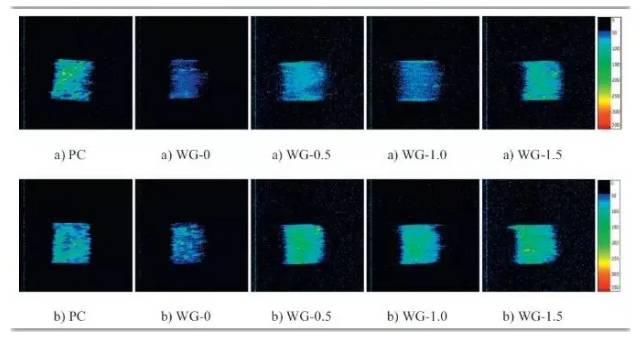
Figure 5. NMR pseudocolor images of samples before and after carbonation
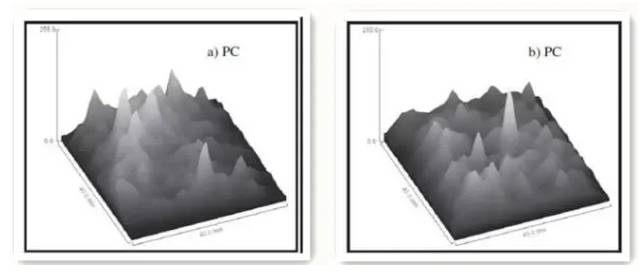
Figure 6-1. NMRI grayscale images of PC mortar before and after carbonation
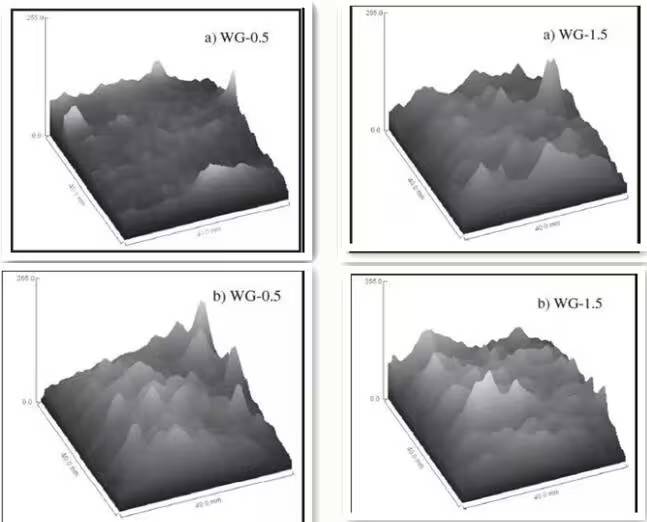
Figure 6-2. Grayscale values of MRI images before and after carbonation
04. Effect of Carbonation on NMR Transverse Relaxation Time (T2)
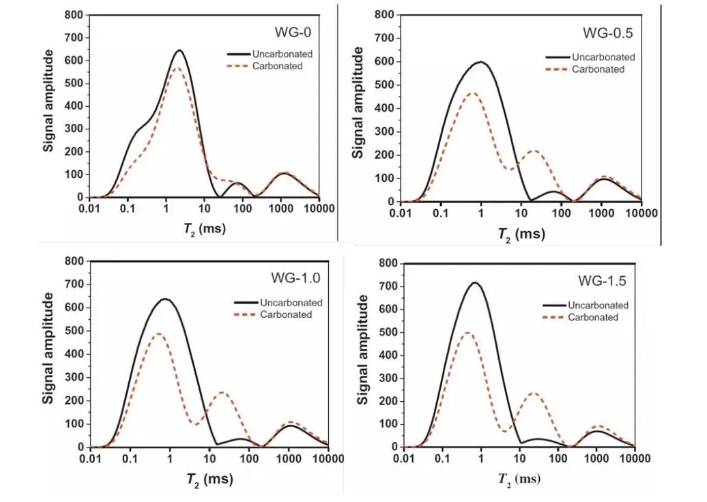
Figure 7. T2 spectra of AAS specimens before and after carbonation at various concentrations
As shown above, significant changes were observed in the T2 relaxation spectra of AAS mortars after carbonation. The number of micropores decreased, while mesopores and macropores increased. The pore size distributions of both AAS and PC mortars were similar at larger scales, and the range of pore sizes reflected the density variation of the reaction products.
Conclusions
(1) Carbonation negatively affects the compressive strength of alkali-activated slag (AAS) mortars or concrete.
(2) Compared to pure NaOH, increasing sodium silicate modulus leads to C-S-H gels with lower Ca/Si ratios, which improves compressive strength. In AAS systems, especially with sodium silicate, calcium is more easily leached from the C-S-H phase.
(3) Low-field NMR enables intuitive and effective analysis of microstructural changes before and after accelerated carbonation, offering a valuable tool for studying the evolution of internal pore structures in AAS and PC materials.
References
[1] Li, Ning, N. Farzadnia, and C. Shi. “Microstructural changes in alkali-activated slag mortars induced by accelerated carbonation.” Cement and Concrete Research 100 (2017): 214–226.
[2] Alkali-Activated Cement and Concrete [M]. Shi Caijun et al. Beijing: Chemical Industry Press, July 2008.
Phone: 400-060-3233
After-sales: 400-060-3233


Back to Top