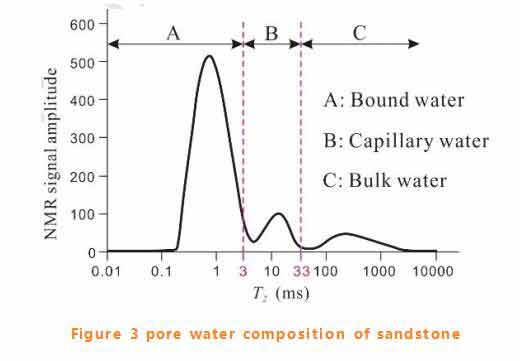
Pore water composition of sandstone
Typical fully saturated sandstone T2 spectrum at room temperature contains three peaks (as shown in figure 3), with A dominated by bound water (76%), B capillary water (14%) and C free water (10%).Because sandstone does not contain clay minerals, bound water is basically adsorbed water. The spectral area ratio of each component represents the relative content of each component in pore water.
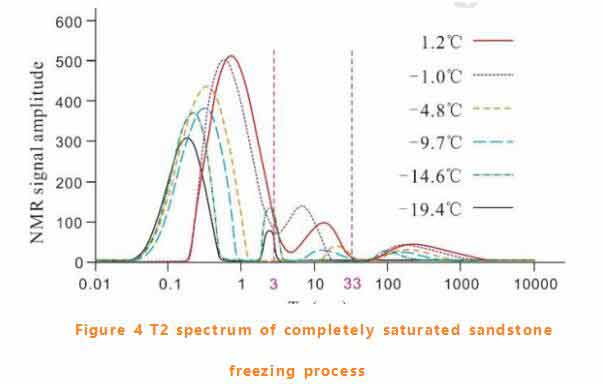
T2 spectrum of sandstone freezing process
The T2 spectrum changes during the freezing process of fully saturated sandstone are shown in figure 4.With the decrease of temperature, the first two peaks shifted significantly to the left, and the width and height of each peak decreased .The total area of T2 spectrum decreased slightly at -1℃, indicating that the pore water was too cold at this temperature. When the temperature drops to -14.6℃, the free water peak shrinks and disappears at -19.4℃.However, the bound water peak and capillary water peak shrink obviously in the initial freezing stage.
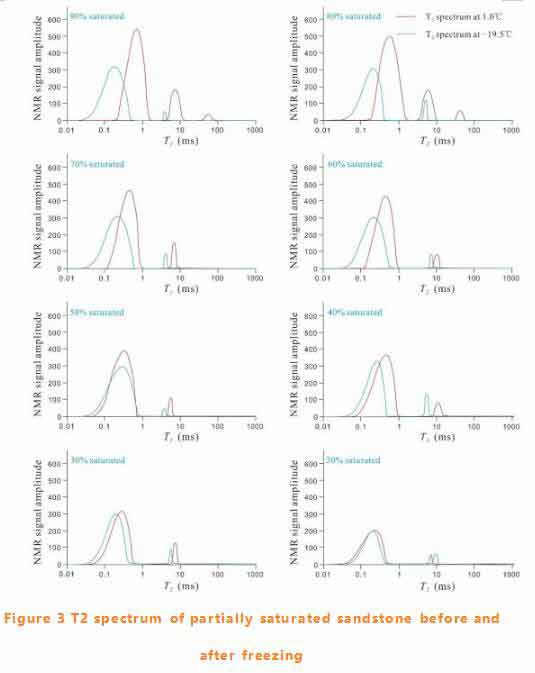
The pore water content of each component is closely related to the initial saturation (see figure 5).The content of irreducible water decreases monotonically with the initial saturation, but the proportion is larger. When the initial saturation is less than 80%, the free water content can be ignored. When saturation < 60%, capillary water is almost unchanged, but the proportion is relatively small.
With the decrease of initial saturation, the T2 spectrum of partially saturated samples gradually shifted to the left before freezing. At -20℃, unfrozen water is basically bound water, capillary water and free water is very little. Time domain NMR When the initial saturation was > 50%, the bound water content of the sample after freezing decreased significantly. When the initial saturation is less than 40%, the bound water content does not change before and after freezing.
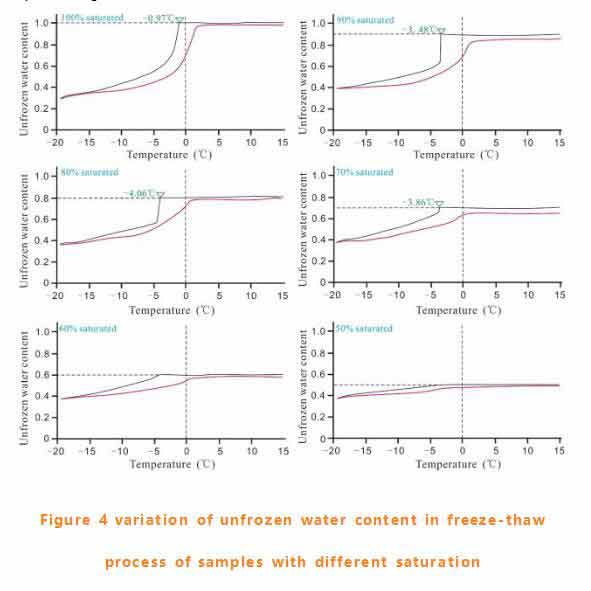
Change of frozen water content in freezing-thawing process
The variation of unfrozen water content of each sample at different temperatures is shown in figure 4.The fully saturated samples still had 40% of the pore water unfrozen. When the saturation of BBB 0 is 70%, the phenomenon of supercooling of pore water is obvious. Supercooling degree increased from 0.97℃(fully saturated sample) to 4.06℃(80% saturated).In addition, pore water content changes with hysteresis during freezing and thawing. Hysteresis rings of samples with low saturation are small, and when the initial saturation is < 50%, the hysteresis rings are almost invisible.
 NIUMAG
NIUMAG